Abstract
Background: There are no randomised trials reporting clinical outcomes of biodegradable polymer biolimus-eluting stents (BP-BES) and durable polymer everolimus-eluting stents (DP-EES) at 10 years.
Aims: We aimed to compare the 10-year clinical outcomes between BP-BES and DP-EES.
Methods: The randomised NOBORI Biolimus-Eluting Versus XIENCE/PROMUS Everolimus-eluting Stent Trial (NEXT) was originally designed to evaluate the non-inferiority of BP-BES relative to DP-EES with the primary efficacy endpoint of target lesion revascularisation (TLR) at 1 year and the primary safety endpoint of death or myocardial infarction (MI) at 3 years. In this extended follow-up study, clinical outcomes were compared from 1 year after stent implantation up to 10 years between patients with BP-BES and DP-EES.
Results: From May to October 2011, NEXT enrolled a total of 3,241 patients from 98 centres in Japan. The current study population consisted of 2,417 patients (1,204 patients with BP-BES and 1,213 with DP-EES) from 66 centres that agreed to participate in the extended study. Complete 10-year follow-up was achieved in 87.5% of patients. The cumulative 10-year incidence of death or MI was 34.0% in the BP-BES group and 33.1% in the DP-EES group (hazard ratio [HR] 1.04, 95% confidence interval [CI]: 0.90-1.20; p=0.58). TLR occurred in 15.9% of patients in the BP-BES group and in 14.1% of the DP-EES group (HR 1.12, 95% CI: 0.90-1.40; p=0.32). In a landmark analysis at 1 year, the cumulative incidences of death or MI and TLR were not significantly different between the 2 groups.
Conclusions: The safety and efficacy outcomes for BP-BES were not significantly different from those for DP-EES at 1 year and up to 10 years after stent implantation.
Introduction
New-generation drug-eluting stents (DES) were developed to overcome the long-term adverse events related to the durable polymer used in the first-generation DES123. The new-generation biocompatible durable polymer everolimus-eluting stents (DP-EES) were reported to be associated with a significantly lower risk for stent thrombosis (ST) as compared with first-generation DES or bare metal stents4. The new-generation biodegradable polymer biolimus-eluting stents (BP-BES) were also reported to have a significantly lower risk for very late stent thrombosis (VLST) as compared with first-generation DES in the Limus Eluted From A Durable Versus ERodable Stent Coating (LEADERS) trial5. In several meta-analyses of randomised clinical trials comparing the safety and efficacy of biodegradable polymer DES (BP-DES) with the new-generation durable polymer DES (DP-DES), BP-DES and DP-DES have demonstrated a similar efficacy and safety profile in the follow-up duration of up to 5 years67. However, there is a scarcity of data on the clinical outcomes of BP-DES relative to new-generation DP-DES beyond 5 years after implantation. Very long-term follow-up is important in evaluating the safety and efficacy profiles of BP-DES compared to DP-DES considering that the occurrence of stent-related adverse events do not attenuate over time28. Therefore, we sought to evaluate the 10-year clinical outcomes of BP-BES as compared with DP-EES in the extended follow-up study of the NOBORI Biolimus-Eluting versus XIENCE/PROMUS Everolimus-eluting Stent Trial (NEXT), which is the largest prospective multicentre randomised trial for this comparison9.
Methods
Study design and patients
NEXT is a prospective, multicentre, randomised, assessor-blind, non-inferiority trial comparing BP-BES with DP-EES in Japan, as previously described in detail910. Written informed consent was obtained from all the study patients. The study was registered at Clinical Trials.gov: NCT01303640. Briefly, patients scheduled for percutaneous coronary intervention (PCI) using DES across 98 participating centres were randomly assigned to undergo PCI with either BP-BES or DP-EES with no exclusion criteria. Randomisation was performed in a 1:1 ratio by a web-based allocation system and was stratified by centre, diabetic status, and participation in the imaging substudies. The study group assignments were blinded to the statistician, members of the independent clinical events committee, the steering committee, the clinical research organisation (Research Institute for Production Development, Kyoto, Japan), the angiographic core laboratory (Cardiocore, Tokyo, Japan) and the sponsor (Terumo Japan) (Supplementary Appendix 1).
The trial was originally designed to evaluate the non-inferiority of BP-BES relative to DP-EES in terms of any target lesion revascularisation (TLR) at 1 year and a composite of death or myocardial infarction (MI) at 3 years, both of which were met and previously reported910. The extended 5-year follow-up study of NEXT has also already been reported11. This extended 10-year follow-up study was planned after completion of the 3-year follow-up of the NEXT Trial, and we enrolled only patients from the centres that agreed to participate in this extended study. The current extended follow-up study was approved by all institutional review boards in the 66 centres that agreed to participate (Supplementary Appendix 2); it was carried out by the opt-out method. Among a total of 3,241 patients from the entire NEXT study population at 98 centres, 2,417 patients (1,204 patients with BP-BES and 1,213 with DP-EES) with 3,020 lesions were included in the extended follow-up study (Figure 1). For the present analysis, the clinical outcomes were compared between the 2 groups at 1 year and up to 10 years after stent implantation.
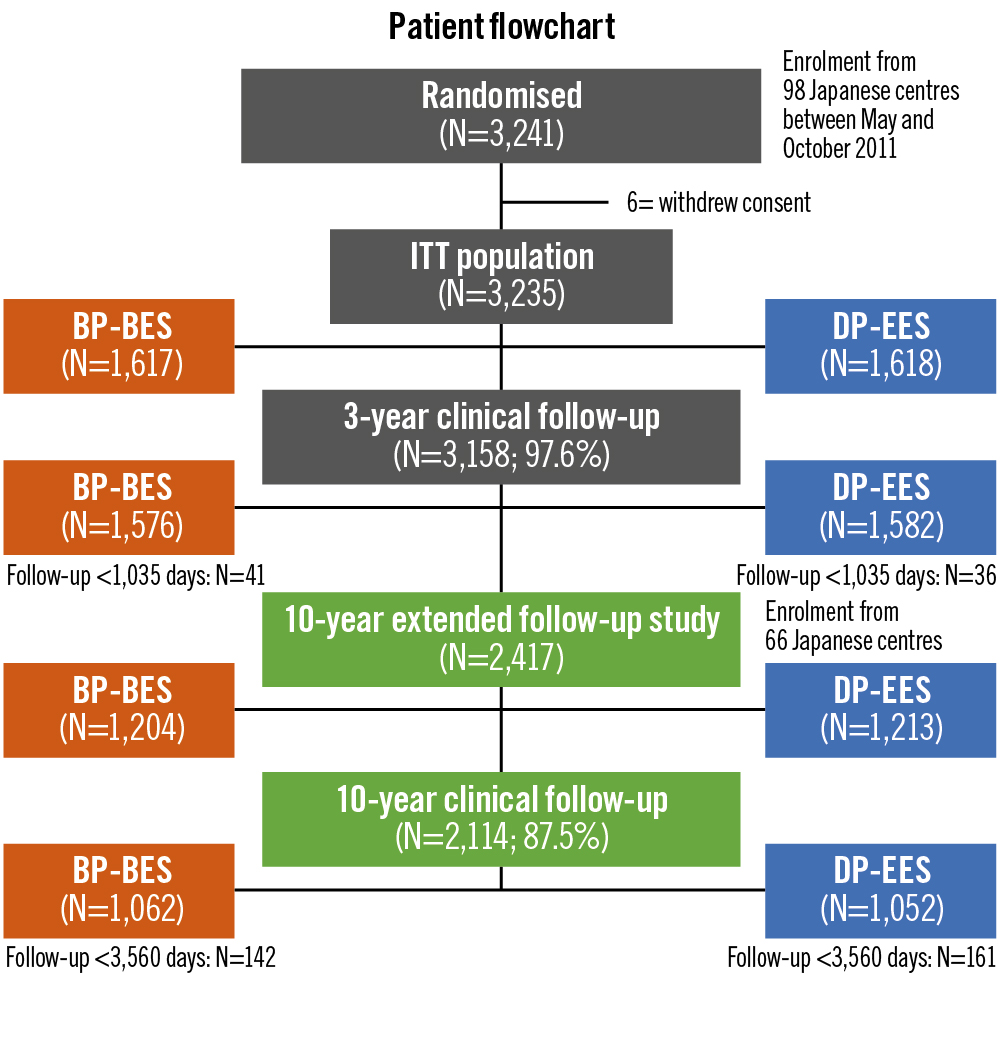
Figure 1. Study patient flowchart. BP-BES: biodegradable polymer biolimus-eluting stent; DP-EES: durable polymer everolimus-eluting stent; ITT: intention-to-treat
Study procedures
Details of the study procedures are described in Supplementary Appendix 3. Baseline and follow-up data were reported, not by the physicians but by the dedicated clinical research coordinators belonging to the participating centres, to local site management organisations or to the clinical research organisation. Most of the follow-up information was obtained from the outpatient hospital chart. For patients without hospital visits, the follow-up information was collected by a mail questionnaire or by a telephone call to the referring physician. All the primary endpoint events were adjudicated by the independent clinical event committee based on the original source documents. For patients with target vessel revascularisation (TVR), angiograms were analysed by the angiographic core laboratory in order to distinguish TLR from TVR.
The recommended antiplatelet regimen included aspirin (≥81 mg daily) indefinitely and thienopyridines (75 mg clopidogrel or 200 mg ticlopidine daily) for at least 3 months. The duration of dual antiplatelet therapy was left to the discretion of each attending physician. The status of antiplatelet therapy was evaluated throughout the follow-up period. Discontinuation of thienopyridines and aspirin was defined to be persistent when it had been withdrawn for more than 2 months1. Discontinuation of dual antiplatelet therapy was defined as persistent discontinuation of either thienopyridines or aspirin.
For the present analysis, the primary efficacy endpoint was any TLR, while the primary safety endpoint was a composite of death or MI. Other prespecified outcome measures included clinically driven TLR, TVR, any coronary revascularisation, all-cause death, cardiac death, MI, ST, hospitalisation for heart failure, stroke, bleeding, a device-oriented composite outcome (cardiac death, target vessel MI or TLR), a patient-oriented composite outcome (all-cause death, MI or any repeat coronary revascularisation), target lesion failure (TLF; cardiac death, target vessel MI or ischaemia-driven TLR), target vessel failure (TVF; cardiac death, MI or ischaemia-driven TVR), and major adverse cardiac events (MACE; cardiac death, MI or ischaemia-driven TLR). Definitions are described in detail in Supplementary Appendix 3.
Statistical analysis
Categorical variables are presented as number and percentage and were compared with the chi-square test. Continuous variables are expressed as mean value±standard deviation or median with interquartile range. Continuous variables were compared using the Student’s t-test or Wilcoxon rank-sum test based on their distributions.
NEXT was a non-inferiority trial, which was powered for evaluating the non-inferiority of BP-BES compared to DP-EES for the primary efficacy endpoint of TLR at 1 year9. With the assumption of a 6.9% TLR rate, a total of 3,000 patients would yield 95% power to detect non-inferiority with a non-inferiority margin of 3.4% (half of the 6.9%) at a level of a 1-sided type 1 error of 0.025. A total of 3,200 patients were to be enrolled, taking into consideration possible dropout during follow-up. With the assumption of a 12.2% rate for the primary safety endpoint of death or MI at 3 years, 3,000 patients would yield 91% power with a non-inferiority margin of 4.3% at a level of a 1-sided type 1 error of 0.025. In the extended follow-up study, only the superiority of BP-BES relative to DP-EES was evaluated.
Clinical outcomes were analysed according to the intention-to-treat principle. Each endpoint was assessed by the Kaplan-Meier method. The effect of treatment was compared using the Cox proportional hazards model and was expressed by hazard ratios (HR) with 95% confidence intervals (CI). P-values for HR were estimated by the Wald method. In the primary efficacy outcome of TLR, the cumulative incidences were calculated by Fine and Gray’s method with death as a competing risk that prevents TLR from happening. Clinical follow-up at 10 years was considered complete with an allowance of 3 months (i.e., at least 3,560 days follow-up). Clinical outcomes between 1 and 10 years were evaluated using the landmark analysis method, in which we set the 1-year landmark point. Clinical outcomes within and beyond 5 years were also evaluated using the landmark analysis method, in which we set the 5-year landmark point. Patients who had the endpoint event within the landmark point were excluded from the landmark analysis for the endpoint of interest. As a subgroup analysis, the treatment effect of BP-BES relative to DP-EES was evaluated in several subgroups, including patients with diabetes, insulin-treated diabetes, the elderly, on haemodialysis, and those who had undergone multivessel PCI and PCI to the left anterior descending artery. In these subgroup analyses, we also conducted a formal interaction test between the stent type and subgroup factors.
All statistical analyses were performed by a physician (M. Natsuaki) and a statistician (T. Morimoto) with the use of JMP 15 (SAS Institute) software, SAS 9.4 (SAS Institute) and EZR 1.52 software (R Foundation for Statistical Computing). All reported p-values were 2-sided, and p-values <0.05 were considered statistically significant.
Results
In baseline clinical characteristics, haemodialysis and nitrate use were more prevalent in the BP-BES group than in the DP-EES group, while patients >75 years and who had received treatment of the left circumflex coronary artery were more prevalent in the DP-EES group than in the BP-BES group (Table 1). Regarding the procedural characteristics, the maximum stent inflation pressure was slightly but significantly higher in the BP-BES group than in the DP-EES group. Regarding lesion characteristics, the in-stent minimum lumen diameter and in-segment percentage diameter stenosis after the procedure were significantly larger in the BP-BES group than in the DP-EES group (Table 1).
Complete 10-year follow-up was achieved in 2,114 patients (87.5%) (Figure 1). The cumulative incidence of persistent discontinuation of dual antiplatelet therapy was not significantly different between the BP-BES and DP-EES groups (15.5% vs 13.9% at 1 year, 62.2% vs 62.2% at 5 years and 80.4% vs 79.6% at 10 years; p=0.61) (Figure 2). The cumulative 10-year incidence of persistent discontinuation of aspirin and thienopyridines was also not significantly different between the BP-BES and DP-EES groups (36.8% vs 36.7%; p=0.85 and 66.4% vs 65.3%; p=0.39, respectively).
The primary safety endpoint of death or MI occurred in 385 patients (34.0%) in the BP-BES group and in 375 patients (33.1%) in the DP-EES group up to 10 years (HR 1.04, 95% CI: 0.90-1.20; p=0.58) (Table 2, Figure 3). Regarding the efficacy endpoint of TLR, the angiographic core laboratory evaluated the angiograms at the time of events in 297 out of 314 first TLR events (94.6%) and in 427 out of 456 first TVR events (93.6%). The cumulative 10-year incidence of any TLR was not significantly different between the BP-BES and DP-EES groups (15.9% vs 14.1%, HR 1.12, 95% CI: 0.90-1.40; p=0.32) (Table 2, Figure 3). With death as a competing risk for TLR, cumulative incidences were calculated. The cumulative 10-year incidence of TLR was not significantly different between the BP-BES and DP-EES groups (14.2% vs 12.8%, HR 1.11, 95% CI: 0.89-1.38; p=0.37). The cumulative incidence of definite ST was very low and similar between the 2 groups (0.87% vs 1.03%; p=0.84). The cumulative incidences of TVR and clinically driven TLR were also not significantly different between the BP-BES and DP-EES groups (Table 2). A sensitivity analysis was conducted in 1,441 lesions treated exclusively with BP-BES and 1,448 lesions treated exclusively with DP-EES. The cumulative incidence of lesion-based TLR among lesions treated exclusively with the study stents was not different between the BP-BES and DP-EES groups (16.6% vs 15.7%; p=0.60) (Supplementary Figure 1). A patient-level sensitivity analysis was also conducted in 1,138 patients in whom all lesions were treated exclusively with BP-BES and 1,156 patients in whom all lesions were treated exclusively with DP-EES. Cumulative incidences were not significantly different between the BP-BES and DP-EES groups in terms of the primary safety and efficacy endpoints (33.5% vs 32.6%; p=0.58 and 15.8% vs 13.8%; p=0.27, respectively) (Supplementary Figure 2).
Between 1 and 10 years, the cumulative incidence of death or MI was not different between the 2 groups (29.8% vs 29.5%; p=0.82) (Figure 4, Supplementary Table 1). The cumulative incidences of both definite VLST and late TLR beyond 1 year were also not significantly different between the 2 groups (0.54% vs 0.94%; p=0.30 and 11.9% vs 9.8%; p=0.14, respectively) (Figure 4, Supplementary Table 1). The cumulative incidence of TVR beyond 1 year was significantly higher in the BP-BES group than in the DP-EES group (18.0% vs 14.1%; p=0.02) (Supplementary Table 1). In the landmark analysis up to and beyond 5 years, the cumulative incidences of death or MI and TLR were also not significantly different between the 2 groups (Supplementary Figure 3).
In the subgroup analysis, the risk for death or MI and TLR was not significantly different between the BP-BES and DP-EES groups in any subgroups. There were no significant interactions between the subgroup factors and the effect of BP-BES relative to DP-EES for both death or MI and TLR (Figure 5).
Table 1. Patient, lesion and procedural characteristics.
| Biolimus-eluting stentN=1,204 | Everolimus-eluting stentN=1,213 | p-value | ||
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, years | 69.0±9.8 | 69.5±9.7 | 0.20 | |
| Age ≥75 years | 362 (30) | 423 (35) | 0.01 | |
| Men | 930 (77) | 932 (77) | 0.81 | |
| Body mass index | 24.1±3.6 (1,199) | 24.2±3.4 (1,207) | 0.78 | |
| Coexisting condition | ||||
| Hypertension | 973 (81) | 999 (82) | 0.33 | |
| Diabetes | 564 (47) | 556 (46) | 0.62 | |
| Insulin-treated diabetes | 135 (11) | 124 (10) | 0.43 | |
| Treated with oral medication only | 303 (25) | 332 (27) | 0.22 | |
| Treated with diet therapy only | 77 (6.4) | 61 (5.0) | 0.15 | |
| Dyslipidaemia | 964 (80) | 972 (80) | 0.97 | |
| ESRD (eGFR<30 mL/min/1.73 m²) not on haemodialysis | 30 (2.5) | 32 (2.6) | 0.82 | |
| Haemodialysis | 91 (7.6) | 61 (5.0) | 0.01 | |
| Atrial fibrillation | 71 (5.9) | 88 (7.3) | 0.18 | |
| Anaemia (haemoglobin <11.0 g/dL) | 163 (14) | 143 (12) | 0.20 | |
| Chronic obstructive pulmonary disease | 27 (2.2) | 28 (2.3) | 0.91 | |
| Malignancy | 81 (6.7) | 96 (7.9) | 0.26 | |
| Cardiac risk factor | ||||
| Current smoker | 213 (18) | 217 (18) | 0.90 | |
| Family history of coronary artery disease | 217 (18) | 203 (17) | 0.40 | |
| Prior myocardial infarction | 357 (30) | 353 (29) | 0.77 | |
| Prior stroke | 118 (9.8) | 131 (11) | 0.42 | |
| Heart failure | 155 (13) | 140 (12) | 0.32 | |
| Peripheral vascular disease | 122 (10) | 139 (11) | 0.29 | |
| Prior PCI | 617 (51) | 615 (51) | 0.79 | |
| Prior coronary artery bypass grafting | 59 (4.9) | 58 (4.8) | 0.89 | |
| Clinical characteristics | ||||
| Clinical presentation | 0.91 | |||
| Stable coronary artery disease | 1,004 (83) | 1,019 (84) | ||
| Unstable angina | 139 (12) | 136 (11) | ||
| Acute myocardial infarction | 61 (5.1) | 58 (4.8) | ||
| Left ventricular ejection fraction <30% | 26 (2.2) | 18 (1.5) | 0.21 | |
| Multivessel disease | 612 (51) | 640 (53) | 0.34 | |
| Target vessel location | ||||
| Left main coronary artery | 37 (3.1) | 39 (3.2) | 0.84 | |
| Left anterior descending coronary artery | 595 (49) | 568 (47) | 0.20 | |
| Left circumflex coronary artery | 285 (24) | 333 (27) | 0.03 | |
| Right coronary artery | 410 (34) | 384 (32) | 0.21 | |
| Bypass graft | 9 (0.8) | 9 (0.7) | 0.99 | |
| Complexity of coronary artery disease | ||||
| No. of treated lesions per patient | 1.25±0.55 | 1.24±0.51 | 0.61 | |
| SYNTAX score | ||||
| Number of patients analysed | 1188 | 1129 | ||
| Median | 10 (6-17) | 10 (6-16) | 0.34 | |
| Tertiles | 0.59 | |||
| Low (≤22) | 988 (88) | 1,002 (89) | ||
| Intermediate (23-32) | 103 (9.2) | 94 (8.3) | ||
| High (≥33) | 27 (2.4) | 33 (2.9) | ||
| Medications | ||||
| Aspirin | 1,203 (99.9) | 1,208 (99.6) | 0.10 | |
| Thienopyridines | 1,200 (99.7) | 1,201 (99.0) | 0.046 | |
| Clopidogrel | 996 (83) | 1,023 (85) | 0.34 | |
| Ticlopidine | 187 (16) | 163 (14) | ||
| Others | 17 (1.4) | 15 (1.3) | ||
| Statins | 939 (78) | 947 (78) | 0.96 | |
| Beta blockers | 459 (38) | 455 (38) | 0.76 | |
| ACEI/ARB | 741 (62) | 772 (64) | 0.29 | |
| Calcium-channel blockers | 569 (47) | 556 (46) | 0.48 | |
| Nitrates | 337 (28) | 291 (24) | 0.03 | |
| Coumadin | 83 (6.9) | 99 (8.2) | 0.24 | |
| Lesion and procedural characteristics | ||||
| Number of lesions treated | 1,511 | 1,509 | ||
| Before index procedure | ||||
| Lesion length, mm | 19.6±13.1 (1,371) | 19.5±13.1 (1,386) | 0.80 | |
| Reference vessel diameter, mm | 2.63±0.59 (1,441) | 2.62±0.56 (1,454) | 0.50 | |
| Minimum lumen diameter, mm | 0.77±0.43 (1,445) | 0.76±0.41 (1,456) | 0.35 | |
| Percentage diameter stenosis, % | 71.2±14.5 (1,445) | 71.3±14.5 (1,456) | 0.77 | |
| Thrombus | 28/1,445 (1.9) | 30/1,456 (2.1) | 0.81 | |
| Chronic total occlusion | 115 (7.6) | 103 (6.8) | 0.40 | |
| In-stent restenosis | 184 (12) | 168 (11) | 0.37 | |
| Culprit for STEMI | 45 (3.0) | 45 (3.0) | 0.99 | |
| Bifurcation | 662/1,446 (46) | 669/1,453 (46) | 0.89 | |
| Moderate or heavy calcification | 307/1,446 (21) | 288/1,456 (20) | 0.33 | |
| Small vessel (reference vessel diameter ≤2.75 mm) | 862/1,441 (60) | 900/1,454 (62) | 0.25 | |
| Long lesion (lesion length >18 mm) | 593/1,371 (43) | 584/1,386 (42) | 0.55 | |
| After index procedure | ||||
| No. of stents used | Per patient | 1.58±0.85 | 1.58±0.84 | 0.93 |
| Per lesion | 1.26±0.61 | 1.27±0.63 | 0.55 | |
| Total stent length, mm | Per patient | 32.9±20.4 | 32.7±21.0 | 0.84 |
| Per lesion | 26.2±15.9 | 26.3±16.8 | 0.87 | |
| Stent diameter, mm | 2.90±0.65 | 2.87±0.64 | 0.22 | |
| Multivessel PCI | 142/1,204 (12) | 135/1,213 (11) | 0.61 | |
| Direct stenting | 305/1,461 (21) | 308/1,465 (21) | 0.92 | |
| Maximum stent inflation pressure, atm | 17.4±4.6 (1,461) | 17.0±4.6 (1,462) | 0.02 | |
| Post-dilatation | 1,083 (72) | 1,066 (71) | 0.53 | |
| Bifurcation 2-stent approach | 22 (1.5) | 18 (1.2) | 0.53 | |
| Intravascular ultrasound use | 1,335 (88) | 1,319 (87) | 0.43 | |
| Received study stent only | 1,441/1,451 (99.3) | 1,448/1,453 (99.7) | 0.19 | |
| Crossover to another stent | 9/1,451 (0.62) | 1/1,453 (0.34) | 0.01 | |
| Received study stent only (patient level) | 1,138/1,204 (94.5) | 1,156/1,213 (94.9) | 0.38 | |
| Minimum lumen diameter, mm | In-stent | 2.51±0.48 (1,443) | 2.47±0.45 (1,448) | 0.03 |
| In-segment | 2.09±0.56 (1,446) | 2.08±0.52 (1,451) | 0.49 | |
| Percentage diameter stenosis, % | In-stent | 10.0±7.9 (1,442) | 10.0±7.9 (1,448) | 0.93 |
| In-segment | 22.0±12.1 (1,445) | 20.9±10.9 (1,451) | 0.01 | |
| Acute gain, mm | In-stent | 1.74±0.50 (1,442) | 1.71±0.50 (1,448) | 0.18 |
| In-segment | 1.32±0.53 (1,445) | 1.32±0.53 (1,451) | 0.97 | |
| Duration of procedure, mins | 71.3±43.8 (1,204) | 69.3±43.4 (1,213) | 0.27 | |
| Successful outcome | ||||
| Lesion success by any treatment modalities | 1,505 (99.6) | 1,499 (99.3) | 0.31 | |
| Lesion success by study stents (acute device success) | 1,445/1,451 (99.6) | 1,449/1,453 (99.7) | 0.53 | |
| Procedural success (patient level) | 1,167/1,204 (96.9) | 1,176/1,213 (97.0) | 0.97 | |
| Staged PCI procedures | 329/1,204 (27.0) | 325/1,213 (26.8) | 0.77 | |
| Data are n or n/N (%), mean±SD (n) or median (IQR). ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blockers; eGFR: estimated glomerular filtration rate; ESRD: end-stage renal disease; IQR: interquartile range; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction; SYNTAX: Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery | ||||
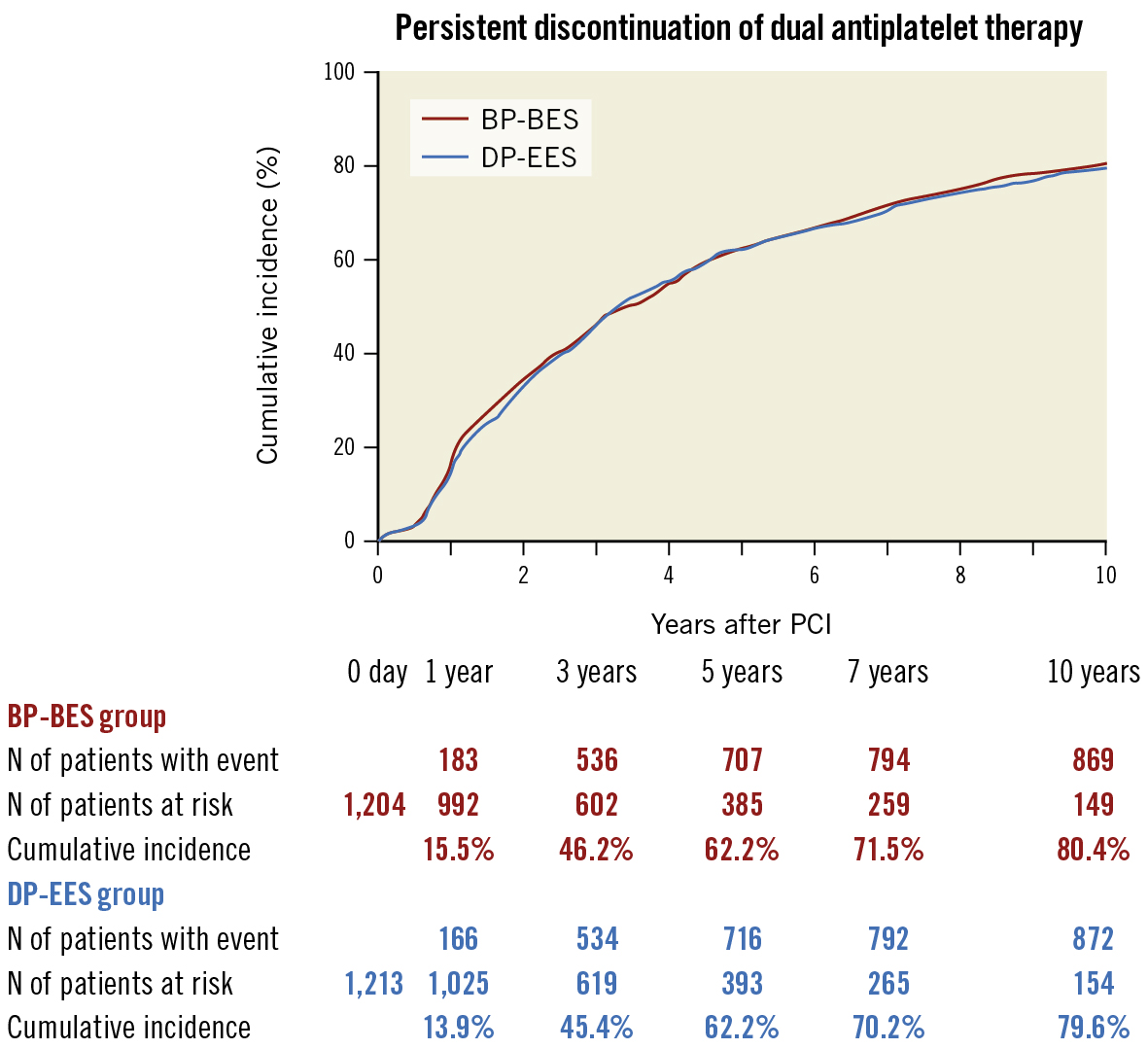
Figure 2. Kaplan-Meier curves for persistent discontinuation of dual antiplatelet therapy (p=0.61). BP-BES: biodegradable polymer biolimus-eluting stent; DP-EES: durable polymer everolimus-eluting stent; PCI: percutaneous coronary intervention
Table 2. Clinical outcomes at 10 years.
| No. of patients with at least 1 event(cumulative incidence) | HR(95% CI) | p-value | |||
|---|---|---|---|---|---|
| Biolimus-eluting stentN=1,204 | Everolimus-eluting stentN=1,213 | ||||
| Death or myocardial infarction | 385 (34.0) | 375 (33.1) | 1.04 (0.90-1.20) | 0.58 | |
| TLR | Any | 164 (15.9) | 150 (14.1) | 1.12 (0.90-1.40) | 0.32 |
| Clinically driven | 134 (13.1) | 121 (11.5) | 1.13 (0.89-1.45) | 0.32 | |
| TVR | Any | 244 (23.5) | 212 (19.9) | 1.19 (0.99-1.43) | 0.06 |
| Clinically driven | 197 (19.2) | 175 (16.6) | 1.15 (0.94-1.42) | 0.16 | |
| Non-TLR | 119 (11.4) | 106 (10.0) | 1.15 (0.89-1.50) | 0.29 | |
| Coronary revascularisation | Any | 400 (37.7) | 379 (35.3) | 1.08 (0.94-1.25) | 0.26 |
| Coronary artery bypass grafting | 27 (2.6) | 30 (2.8) | 0.91 (0.54-1.53) | 0.73 | |
| Death | All-cause | 340 (30.3) | 317 (28.1) | 1.10 (0.94-1.28) | 0.24 |
| Cardiac | 117 (11.7) | 101 (10.1) | 1.18 (0.91-1.54) | 0.22 | |
| Myocardial infarction | Any | 81 (7.6) | 78 (7.5) | 1.05 (0.77-1.43) | 0.76 |
| Q-wave | 19 (1.8) | 24 (2.4) | 0.80 (0.44-1.47) | 0.47 | |
| Target vessel | 47 (4.2) | 48 (4.3) | 0.99 (0.66-1.48) | 0.95 | |
| Fatal | 10 (0.95) | 5 (0.45) | 2.03 (0.69-5.93) | 0.20 | |
| Hospitalisation for heart failure | 94 (9.0) | 118 (11.2) | 0.80 (0.61-1.05) | 0.11 | |
| Stroke | Any | 92 (9.0) | 108 (10.4) | 0.86 (0.65-1.14) | 0.30 |
| Ischaemic | 71 (7.2) | 75 (7.3) | 0.96 (0.70-1.33) | 0.81 | |
| Haemorrhagic | 23 (2.1) | 34 (3.3) | 0.69 (0.40-1.17) | 0.16 | |
| Bleeding | TIMI major | 79 (7.6) | 92 (8.9) | 0.87 (0.65-1.18) | 0.38 |
| TIMI minor/major | 109 (10.4) | 117 (11.2) | 0.95 (0.73-1.23) | 0.71 | |
| TIMI minimal/minor/major | 165 (15.4) | 182 (16.9) | 0.92 (0.75-1.14) | 0.45 | |
| GUSTO severe | 75 (7.2) | 79 (7.6) | 0.97 (0.71-1.33) | 0.85 | |
| GUSTO moderate/severe | 105 (9.9) | 115 (10.9) | 0.93 (0.71-1.21) | 0.60 | |
| Device-oriented composite endpoint | 290 (27.2) | 269 (25.0) | 1.10 (0.93-1.30) | 0.26 | |
| Patient-oriented composite endpoint | 660 (57.3) | 637 (55.0) | 1.06 (0.95-1.18) | 0.28 | |
| TLF | 263 (24.9) | 244 (22.9) | 1.10 (0.92-1.31) | 0.29 | |
| TVF | 332 (31.2) | 308 (28.8) | 1.11 (0.95-1.29) | 0.19 | |
| MACE | 282 (26.6) | 264 (24.9) | 1.09 (0.92-1.29) | 0.30 | |
| Stent thrombosis | Definite | 9 (0.87) | 10 (1.03) | 0.91 (0.37-2.25) | 0.84 |
| Probable | 0 (0) | 0 (0) | NA | NA | |
| Possible | 47 (4.6) | 42 (4.1) | 1.08 (0.63-1.85) | 0.54 | |
| Definite or probable | 9 (0.87) | 10 (1.03) | 0.91 (0.37-2.25) | 0.84 | |
| Definite, probable or possible | 56 (5.4) | 52 (5.1) | 1.09 (0.75-1.60) | 0.64 | |
| Data are n (%). CI: confidence interval; GUSTO: Global Utilization of Streptokinase and Tissue plasminogen activator for Occluded coronary arteries; HR: hazard ratio; MACE: major adverse cardiac event; TIMI: Thrombolysis in Myocardial Infarction; TLF: target lesion failure; TLR: target lesion revascularisation; TVF: target vessel failure; TVR: target vessel revascularisation | |||||
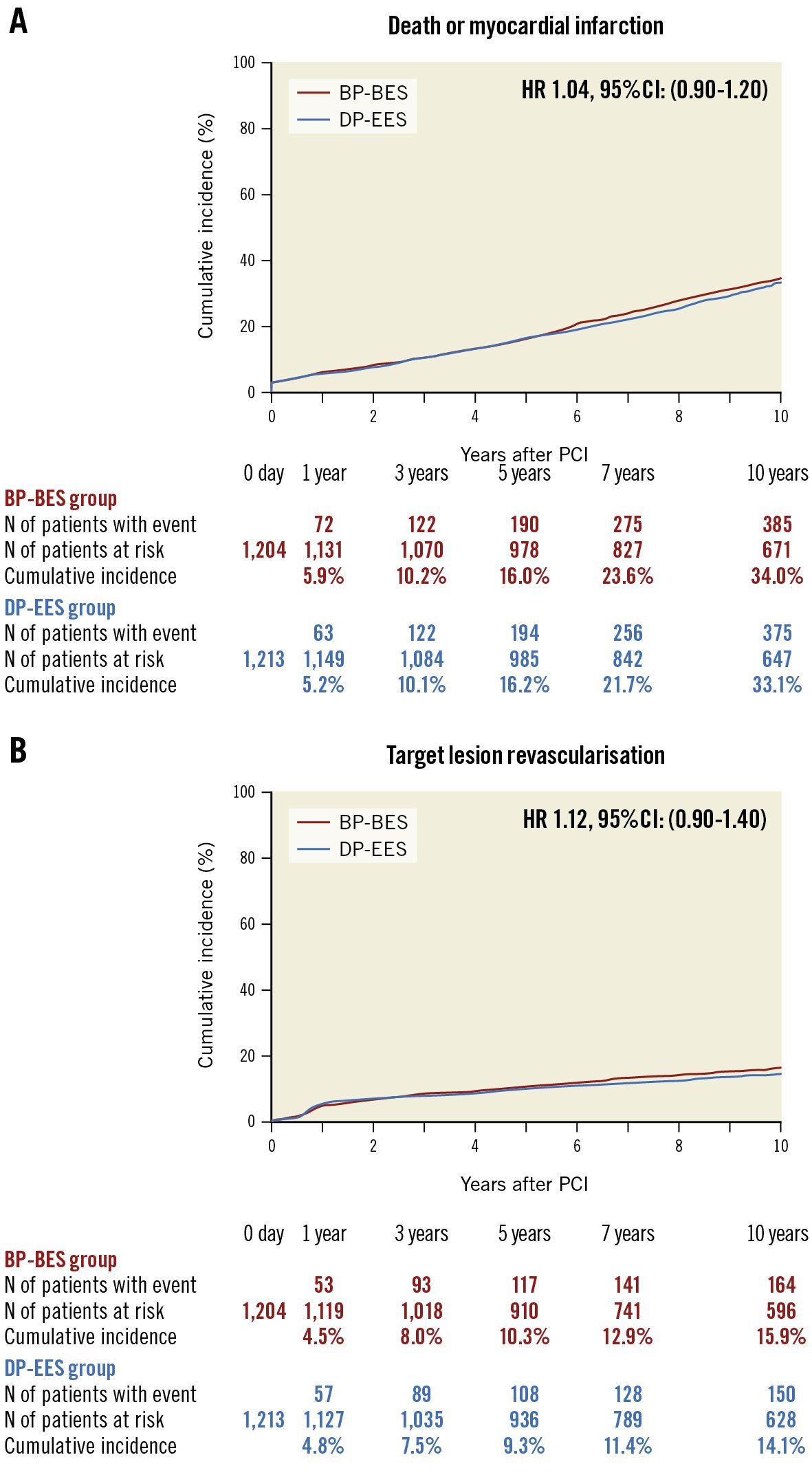
Figure 3. Kaplan-Meier curves for the primary safety and efficacy endpoints up to 10-year follow-up. A) Death or myocardial infarction (p=0.58) and B) target lesion revascularisation (p=0.32). BP-BES: biodegradable polymer biolimus-eluting stent; CI: confidence interval; DP-EES: durable polymer everolimus-eluting stent; HR: hazard ratio; PCI: percutaneous coronary intervention
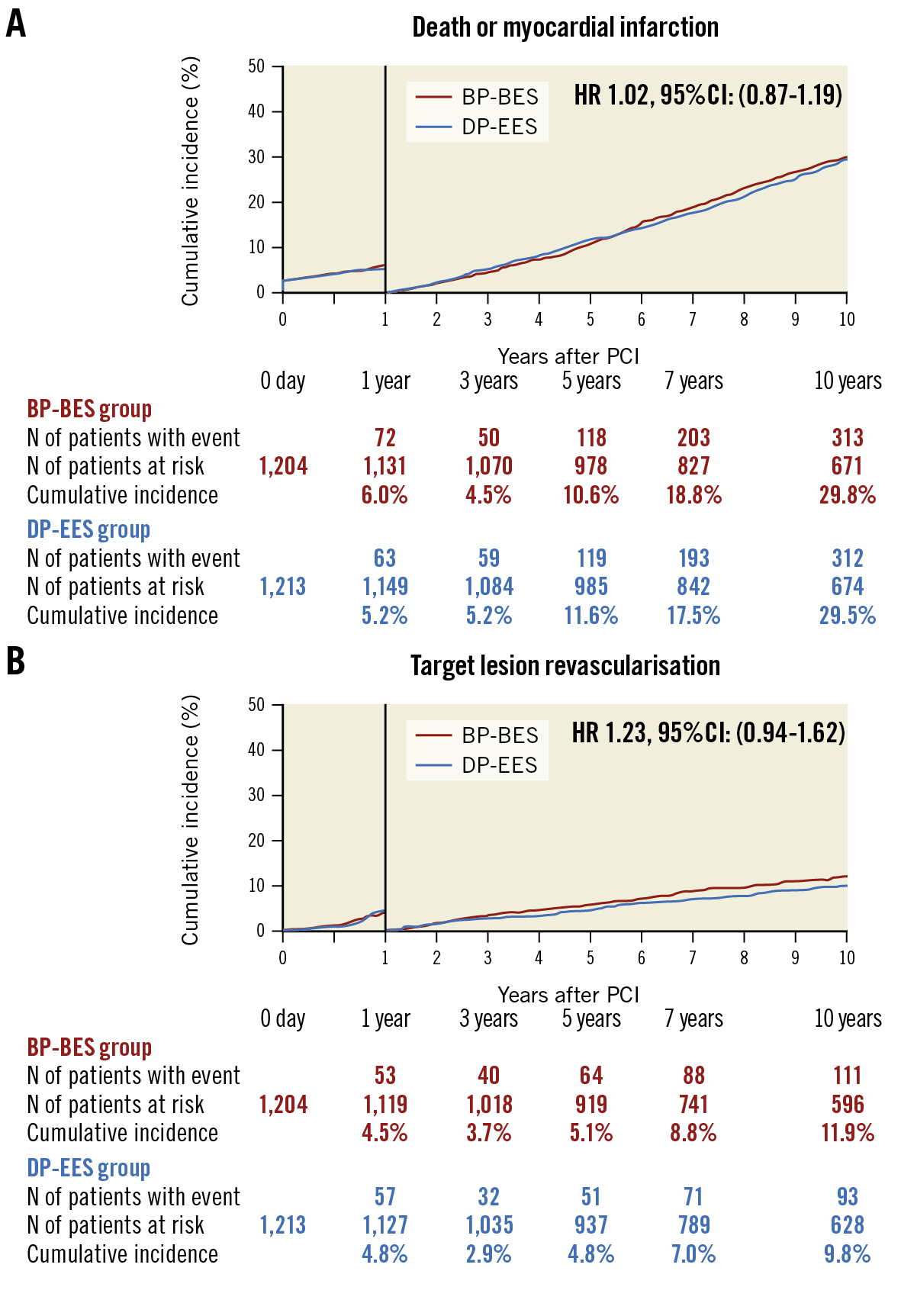
Figure 4. Kaplan-Meier curves for the primary safety and efficacy endpoints between 1 and 10 years by the 1-year landmark analysis. A) Death or myocardial infarction (within 1 year; p=0.41, between 1 and 10 years; p=0.82) and B) target lesion revascularisation (within 1 year; p=0.72, between 1 and 10 years; p=0.14). BP-BES: biodegradable polymer biolimus-eluting stent; CI: confidence interval; DP-EES: durable polymer everolimus-eluting stent; HR: hazard ratio; PCI: percutaneous coronary intervention
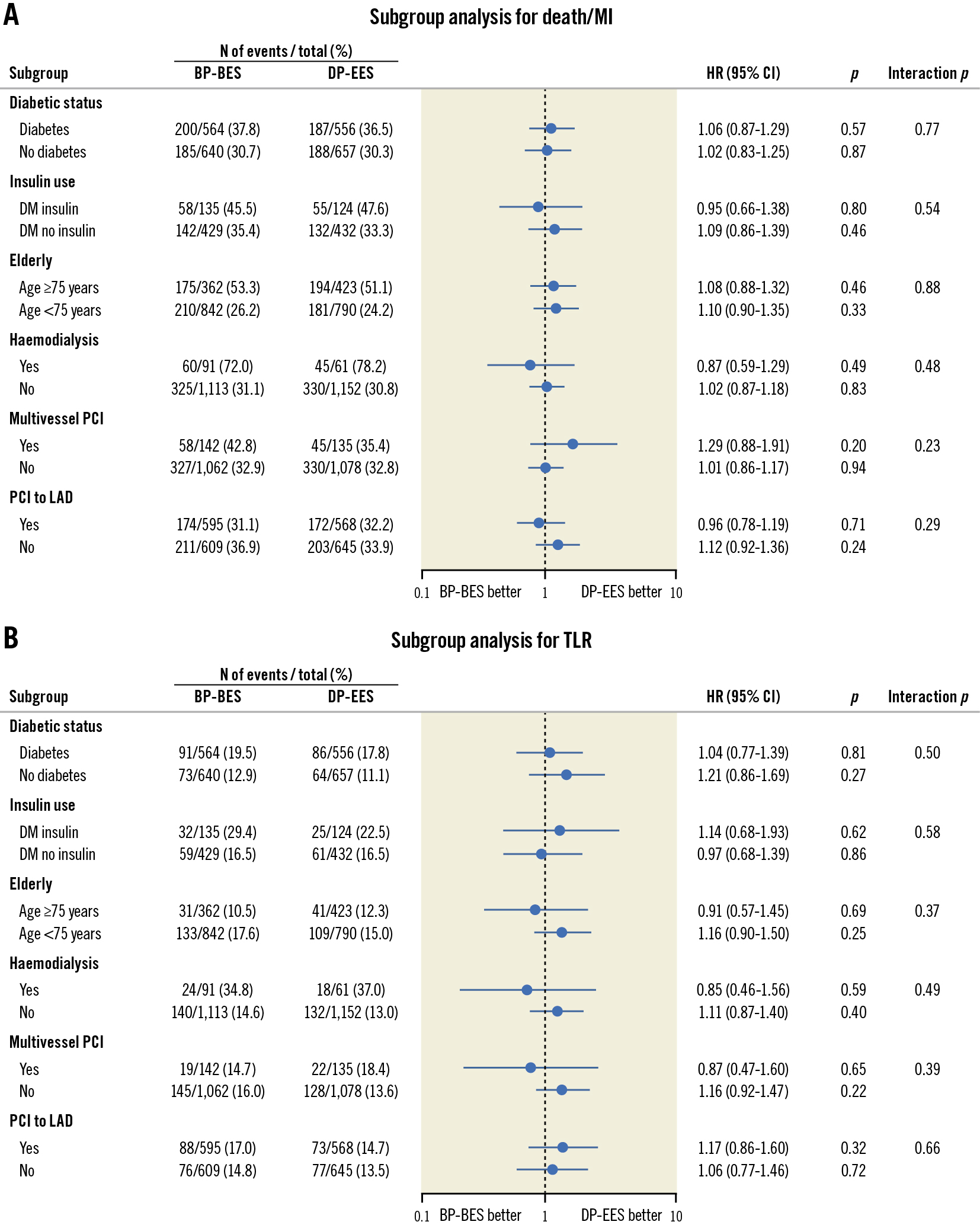
Figure 5. Hazard ratio plots for the primary safety and efficacy endpoints in the subgroups. A) Death or myocardial infarction and B) target lesion revascularisation. BP-BES: biodegradable polymer biolimus-eluting stent; CI: confidence interval; DM: diabetes mellitus; DP-EES: durable polymer everolimus-eluting stent; HR: hazard ratio; LAD: left anterior descending artery; MI: myocardial infarction; PCI: percutaneous coronary intervention; TLR: target lesion revascularisation
Discussion
The main finding of the current study was that safety and efficacy outcomes of BP-BES were not significantly different from those of DP-EES from 1 year up to 10 years after stent implantation.
There has only been one previous randomised trial evaluating 10-year clinical outcomes of BP-DES relative to DP-DES. In the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents Trial (ISAR-TEST 4), the safety of biodegradable polymer sirolimus-eluting stents (BP-SES; Yukon Choice PC [Translumina Therapeutics]) was compared with DP-EES (XIENCE [Abbott]) and an early-generation durable polymer sirolimus-eluting stent (DP-SES; CYPHER [Cordis]) at 10-year follow-up12. The present 10-year results from NEXT were consistent with those of ISAR-TEST 4 in terms of safety outcomes between patients with BP-SES and DP-EES. In ISAR-TEST 4, the 10-year incidence of all-cause death (BP-SES 31.8% vs DP-EES 30.3%, HR 1.05, 95% CI: 0.88-1.26) was not significantly different between BP-SES and DP-EES recipients. The 10-year incidence of MI (BP-SES 7.7% vs DP-EES 7.9%, HR 0.98, 95% CI: 0.69-1.41) was also similar between patients with BP-SES and DP-EES. On the other hand, the 10-year incidence of all-cause death in early-generation DP-SES patients was significantly higher than in the new-generation BP-SES and DP-EES patients in ISAR-TEST 4 (DP-SES 37.1% vs BP-SES 31.8% and DP-EES 30.3%; p=0.02). Taken together, new-generation DES using biodegradable polymer and durable polymer would likely have similar safety outcomes up to 10 years, while the risk for safety outcomes could be higher in patients with early-generation DES relative to new-generation DES.
The efficacy of BP-DES was also compared with DP-DES up to 10 years in ISAR-TEST 4. The 10-year incidence of TLR (BP-SES 20.3% vs DP-EES 18.2%, HR 1.10, 95% CI: 0.87-1.38) was not significantly different between BP-SES and DP-EES recipients. In line with this report, the primary efficacy endpoint of TLR was not significantly different between BP-BES and DP-EES patients in the current study. However, the cumulative 10-year incidence of TLR was numerically higher in the BP-BES group than in the DP-EES group, especially beyond 1 year after stent implantation. The Nobori BP-BES (Terumo) used in the NEXT Trial has relatively thick struts (120 μm) as compared with the DP-EES (81 μm), which could be one of the reasons for this result. Newer-generation ultrathin-strut DES were compared with older, second-generation thicker-strut DES in the meta-analysis13. In comparison with thicker-strut second-generation DES, newer-generation ultrathin-strut DES were associated with a 16% reduction in target lesion failure (relative risk, 0.84, 95% CI: 0.72-0.99). Therefore, a comparison of thin-strut BP-DES with new-generation DP-DES would be important to assess the true effect of polymer biodegradation on clinical outcomes. In the Clinical Evaluation of New Terumo Drug-Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease Trial (CENTURY II), the Ultimaster BP-SES (Terumo) showed similar efficacy profiles to the XIENCE DP-EES up to 5-year follow-up after PCI14. In the Randomized Comparison of a Sirolimus-eluting Stent With Biodegradable Polymer Versus an Everolimus-eluting Stent With Durable Polymer for Percutaneous Coronary Revascularization trial (BIOSCIENCE), the 5-year clinical outcomes of patients with the Orsiro BP-SES (BIOTRONIK) with ultrathin struts were not significantly different from those of patients with XIENCE DP-EES with respect to the efficacy endpoint, i.e., TLR or target lesion failure15. These results might suggest that clinical outcomes of patients with BP-DES with thin struts are similar to those of patients with new-generation DP-DES.
The effects of biodegradable polymer relative to durable polymer on clinical outcomes should also be evaluated among stents with the similar stent platforms and using similar drugs. In the Prospective Multicenter Trial to Assess the Safety and Effectiveness of the SYNERGY Everolimus-Eluting Platinum Chromium Coronary Stent System (SYNERGY Stent System) for the Treatment of Atherosclerotic Lesion (EVOLVE II), the SYNERGY BP-EES (Boston Scientific) demonstrated comparable outcomes to the PROMUS Element Plus DP-EES (Boston Scientific)16. The 5-year target lesion failure rate was 14.3% for the SYNERGY group and 14.2% for the PROMUS Element Plus group (p=0.91). A landmark analysis demonstrated similar rates of target lesion failure from 1 to 5 years (p=0.94). The rates of clinically indicated TLR or definite/probable ST were not different between the 2 groups, suggesting that both biodegradable polymer and durable polymer DES using a similar stent platform and the same drug have comparable efficacy outcomes after stent implantation up to 5 years. Very long-term follow-up study of thin-strut BP-DES relative to DP-DES up to 10 years is necessary to evaluate the potential benefits of thin-strut BP-DES over DP-DES.
Limitations
Some limitations must be considered when interpreting our results. First, the study population was reduced from 3,235 patients to 2,414 patients in the current extended follow-up study. Patients from only 66 out of the 98 centres that agreed to participate were actually enrolled in this study, hence, selection bias cannot be fully denied. Second, patients participating in the current trial were not fully monitored and, therefore, underreporting of adverse events, such as myocardial infarction or ST, could be possible. However, angiograms in patients with TVR were rigorously evaluated for the presence of thrombus in the angiographic core laboratory, and adjudication of death and MI events was conducted very carefully to evaluate the possibility of ST. Third, despite the all-comer design, predominantly stable patients were included in this study, which may reflect a selection bias. Therefore, the results of this study may not be generalisable to patients with acute coronary syndrome. Fourth, as the Nobori BP-BES is no longer available in most jurisdictions, the results of this study are not applicable to those undergoing PCI in current clinical practice. Moreover, patients were enrolled in 2011, and many medical and interventional treatments for ischaemic heart disease have changed since then. Finally, only a Japanese population was included in this randomised trial, and therefore, the results of this study cannot be extrapolated and generalised to different populations.
Conclusions
The safety and efficacy outcomes of BP-BES patients were not significantly different from those of DP-EES patients at 1 year and up to 10 years after stent implantation.
Impact on daily practice
The NEXT Trial is the first randomised trial reporting 10-year clinical outcomes after biodegradable polymer biolimus-eluting stent (BP-BES) and durable polymer everolimus-eluting stent (DP-EES) implantation. The primary safety endpoint of death or myocardial infarction and the primary efficacy endpoint of target lesion revascularisation were not significantly different between the BP-EES and DP-EES groups from 1 year after stent implantation up to 10 years. Any advantages of BP-BES were not apparent even at 10-year follow-up after stenting.
Acknowledgements
We appreciate the effort of the members of the cardiac catheterisation laboratory and clinical research coordinators in the participating centres.
Funding
This study was funded by Terumo Japan.
Conflict of interest statement
Y. Morino reports being an advisory board member at Terumo Japan and Abbott Medical. M. Natsuaki received honorarium from Terumo and Abbott Medical. K. Tanabe was an advisory board member for and received honorarium from Terumo and Abbott Medical. K. Kozuma reports serving as an advisory board member for Terumo Japan and Abbott Medical; and received honorarium for lectures from Boston Scientific, Abbott Medical, Medtronic, and Terumo. T. Morimoto received lecturer’s fees from AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Japan Lifeline, Kowa, Pfizer, and Tsumura; manuscript fees from Bristol-Myers Squibb and Pfizer; and was on the advisory board for Novartis and Teijin. Y. Nakagawa received honorarium from Terumo and Abbott Medical; and ascholarship donation from Abbott Medical. T. Akasaka received grant/research support from Abbott Medical, Boston Scientific Japan, Daiichi Sankyo, Nipro, and Terumo; consulting fees/honoraria from Abbott Medical, Daiichi Sankyo, Nipro, and Terumo; and served as amedical advisor for Terumo. T. Kimura reports receiving research grants from Abbott Medical and Boston Scientific; honoraria from Abbott Medical, Boston Scientific, Daiichi Sankyo, Sanofi, and Terumo; and serving as an advisory board member for Abbott Medical, Boston Scientific, Sanofi and Terumo Japan. The other authors do not have any conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

