
Introduction
In the meta-analysis of randomised clinical trials comparing the safety and efficacy of a biodegradable polymer drug-eluting stent (BP-DES) as compared with new-generation durable polymer drug-eluting stents (DP-DES), no significant differences were seen between BP-DES and DP-DES with a mean follow-up duration of 26 months1. However, longer-term follow-up would be required to evaluate the safety and efficacy profiles of BP-DES compared to DP-DES considering the occurrence of stent-related adverse events not attenuating over time. Therefore, we sought to evaluate the five-year clinical outcomes of a biodegradable polymer biolimus-eluting stent (BP-BES) as compared with new-generation durable polymer everolimus-eluting stents (DP-EES) in the extended follow-up study from NEXT (NOBORI Biolimus-Eluting versus XIENCE/PROMUS Everolimus-eluting Stent Trial)2.
Methods
STUDY DESIGN, PATIENTS AND PROCEDURES
As previously described in detail, NEXT is a prospective, multicentre, randomised, non-inferiority trial comparing BP-BES with DP-EES in Japan2. Written informed consent was obtained from all the study patients. The study was registered at ClinicalTrials.gov (NCT01303640). The extended follow-up study of NEXT was designed with planned follow-up up to 10 years. All the centres were invited to participate in the extended study, but 20 centres refused to participate in the extended study (Supplementary Appendix 1, Supplementary Appendix 2). Among a total of 3,241 patients for the entire NEXT study population from 98 centres, 2,568 patients (BP-BES 1,283 patients and DP-EES 1,285 patients) with 3,229 lesions were included in the extended follow-up study (Supplementary Figure 1). These 2,568 patients represent 79.2% of the original patient population of the NEXT trial.
Details of the study procedures have been described previously2. For the present analysis, the primary efficacy endpoint was any target lesion revascularisation (TLR), while the primary safety endpoint was a composite of death or myocardial infarction (MI).
STATISTICAL ANALYSIS
In the extended follow-up study, the non-inferiority margin for the primary safety and efficacy endpoints was set as a hazard ratio of 1.38 for the observed event rate in the DP-EES group3. The study protocol was updated in line with this amendment. The present analysis would yield 99% power to detect non-inferiority for the primary safety endpoint and 87% power to detect non-inferiority for the primary efficacy endpoint at a one-sided alpha level of 0.025.
Results
The two groups of patients were generally well balanced in terms of baseline clinical and lesion characteristics (Supplementary Table 1).
Complete five-year follow-up was achieved in 2,408 patients (93.8%) (Supplementary Figure 1). The cumulative incidence of persistent discontinuation of dual antiplatelet therapy (DAPT) was not significantly different between the BP-BES and DP-EES groups (15.3% versus 14.2% at one year, and 61.5% versus 62.8% at five years, p=0.74) (Supplementary Figure 2). The primary safety endpoint of death/MI occurred in 190 patients (15.1%) in the BP-BES group, and in 208 patients (16.5%) in the DP-EES group up to five years, demonstrating non-inferiority of BP-BES to DP-EES (hazard ratio [HR] 0.91, 95% confidence interval [CI]: 0.75-1.11), demonstrating non-inferiority of BP-BES to DP-EES in terms of death/MI (p for non-inferiority <0.0001). Testing for superiority was not statistically significant (p for superiority=0.37) (Table 1, Supplementary Table 2, Figure 1A). The primary efficacy endpoint of TLR occurred in 9.8% in the BP-BES group and in 9.3% in the DP-EES group, demonstrating non-inferiority of BP-BES to DP-EES (HR 1.04, 95% CI: 0.8-1.34), demonstrating non-inferiority of BP-BES to DP-EES in terms of TLR (p for non-inferiority=0.01). Testing for superiority was not statistically significant (p for superiority=0.79) (Table 1, Supplementary Table 2, Figure 1B). A sensitivity analysis was conducted in 3,235 initially randomised subjects of this trial. The cumulative five-year incidences of death or MI and TLR were not significantly different between the two groups (14.8% versus 16.2%, p=0.36 and 9.6% versus 8.7%, p=0.5, respectively) (Supplementary Figure 3).
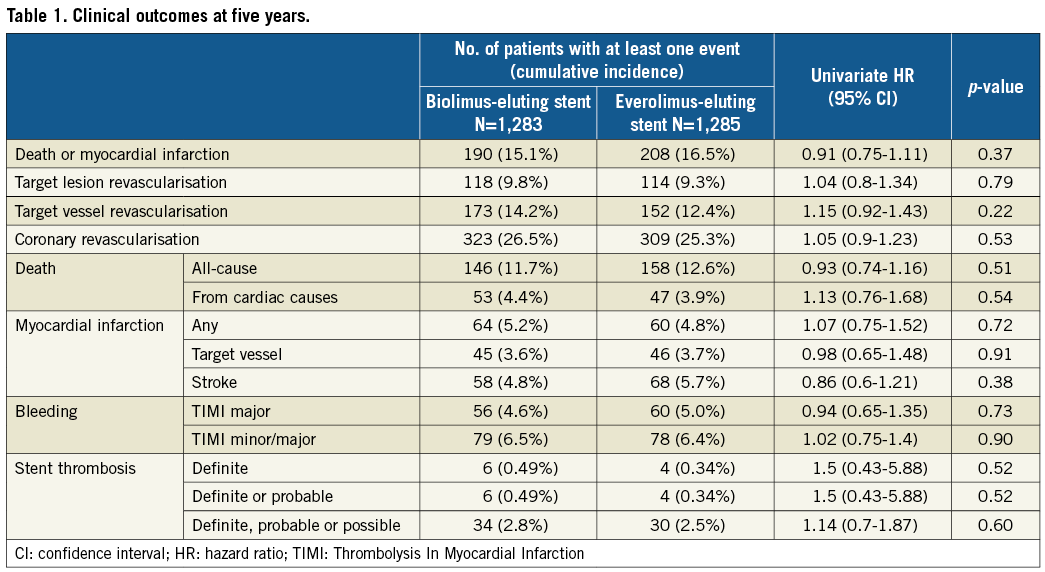
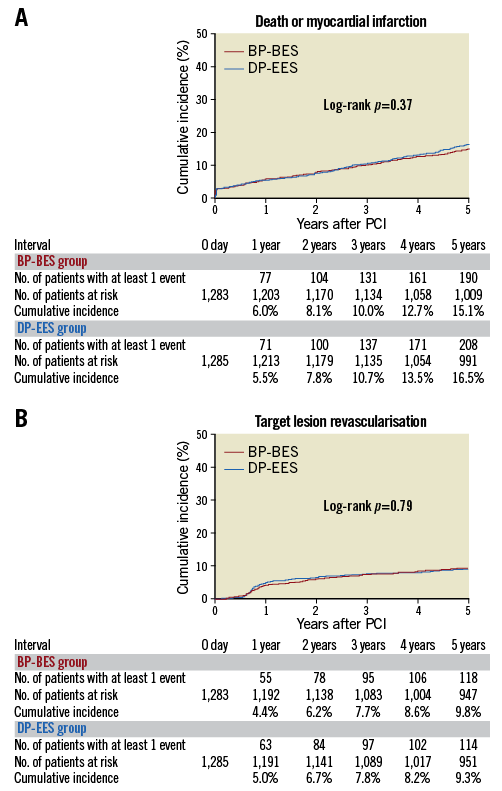
Figure 1. Cumulative incidence of the primary endpoint events up to five-year follow-up. A) Death or myocardial infarction. B) Target lesion revascularisation. BP-BES: biodegradable polymer biolimus-eluting stent; DP-EES: durable polymer everolimus-eluting stent; PCI: percutaneous coronary intervention
Between one and five years, the cumulative incidences of death/MI and TLR were not different between the two groups (Figure 2). The cumulative incidence of definite stent thrombosis (ST) was not different between the two groups (Supplementary Table 3).
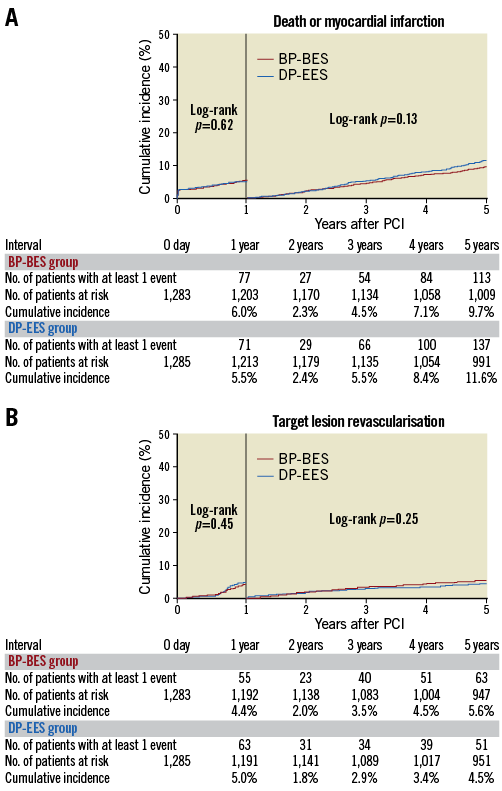
Figure 2. Cumulative incidence of the primary endpoint events between one and five years by one-year landmark analysis. A) Death or myocardial infarction. B) Target lesion revascularisation. BP-BES: biodegradable polymer biolimus-eluting stent; DP-EES: durable polymer everolimus-eluting stent; PCI: percutaneous coronary intervention
In the subgroup analysis, the risk for death/MI and TLR was not significantly different between the BP-BES and DP-EES groups in any pre-specified subgroup (Supplementary Figure 4).
Discussion
The present study is the third randomised trial reporting five-year clinical outcomes between BP-DES versus new-generation DP-DES following the ISAR-TEST 4 and COMPARE II trials4,5. The present five-year results from NEXT were fully consistent with those previous trials4,5. Taken together, new-generation DES using biodegradable polymer and durable polymer would have similar safety and efficacy outcomes up to five years. Both biodegradable polymer and durable polymer might have achieved parallel improvements using more biocompatible polymer than used in first-generation DES. A very long-term follow-up study of BP-DES relative to DP-DES up to 10 years would also provide important information on the potential advantages of BP-DES over DP-DES.
Limitations
First, the number of study participants was reduced from 3,235 patients to 2,568 patients in the current extended follow-up study. However, the main reason for the reduced number of study patients was not incomplete follow-up, but the dropout of 20 centres. Centre was incorporated as one of the stratification factors for randomisation. Therefore, we believe that the reduction in the number of study participants did not have much influence on the study conclusion. Second, DAPT duration was longer than that reported outside Japan. Based on our findings, we cannot exclude that other BP-DES might show a better long-term outcome than DP-DES in the future.
Conclusion
Safety and efficacy outcomes of Nobori® BP-BES (Terumo Corp., Tokyo, Japan) were non-inferior to those of XIENCE/PROMUS DP-EES (Abbott Vascular, Santa Clara, CA, USA, and Boston Scientific, Marlborough, MA, USA, respectively) five years after stent implantation. Advantages of Nobori BP-BES over DP-EES were not apparent even at five-year follow-up after stent implantation.
| Impact on daily practice There is a scarcity of data on the clinical outcomes of BP-BES relative to DP-EES beyond three years after stent implantation. Advantages of BP-BES over current-generation DP-EES were not apparent up to five years and beyond one year after stent implantation. |
Acknowledgements
We appreciate the effort of the members of the cardiac catheterisation laboratory and clinical research co-ordinators in the participating centres.
Funding
Terumo Japan.
Conflict of interest statement
T. Kimura, Y. Morino, K. Tanabe, and K. Kozuma were advisory board members of Terumo Japan and Abbott Vascular Japan. K. Kozuma has received research grant and lecture fees from Abbott Vascular Japan. T. Akasaka has received laboratory funding, a grant, consulting fees and lecture fees from Abbott Vascular Japan. K. Tanabe, Y. Nakagawa and M. Natsuaki have received lecture fees from Abbott Vascular Japan and Terumo Japan. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Study organisation.
Supplementary Appendix 2. List of the participating centres and investigators.
Supplementary Table 1. Patient, lesion and procedural characteristics.
Supplementary Table 2. Clinical outcomes at 5 years.
Supplementary Table 3. Clinical outcomes between 1 year and 5 years.
Supplementary Figure 1. Study patient flow.
Supplementary Figure 2. Cumulative incidence of persistent discontinuation of dual antiplatelet therapy.
Supplementary Figure 3. Cumulative incidences of the primary safety and efficacy endpoint events up to 5-year follow-up in the original entire study population of 3,235 patients.
Supplementary Figure 4. Hazard ratio plot for the primary safety and efficacy endpoints in the pre-specified subgroups.
To read the full content of this article, please download the PDF.

