
“The fundamental things apply, as time goes by” Herman Hupfeld (1894-1951)
Since their advent in clinical practice in 2002, drug-eluting stents (DES) have remarkably improved clinical outcomes of patients undergoing percutaneous coronary intervention (PCI), primarily by almost eliminating the risk of restenosis as compared to bare metal stents1. The treatment effect of early-generation DES, however, came at the expense of delayed arterial healing within the treated vessel, with a subsequently increased risk of stent thrombosis (ST) occurring after the cessation of dual antiplatelet therapy – namely very late ST2. Device iterations have been implemented in order to address this limitation by improving biocompatibility3. Biodegradable polymer-based DES were developed based on evidence of local inflammation within the arterial wall related to persistence of durable polymer coatings during long-term follow-up in patients experiencing ST. The Nobori® biolimus-eluting stent (BES; Terumo, Tokyo, Japan) was one of the first biodegradable polymer-based DES introduced into clinical practice in Europe.
In this issue of EuroIntervention, Jakobsen and co-authors report the final five-year follow-up of the SORT-OUT V trial4, in which all-comer patients undergoing PCI were randomly allocated to the Nobori BES or the CYPHER® sirolimus-eluting stent (SES; Cordis, Cardinal Health, Milpitas, CA, USA) – the efficacy gold standard among early-generation DES.
The trial is part of the SORT-OUT trial series, which is based on data acquired through Danish national registries. The primary findings of SORT-OUT V, reported in 2013, failed to show the hypothesis of non-inferiority of Nobori BES as compared to CYPHER SES in terms of the composite endpoint of cardiac death, myocardial infarction (MI), target vessel revascularisation, and definite ST at nine months (4.1% vs. 3.1%, p for non-inferiority=0.06)5. Since the benefits of biodegradable polymer coatings are expected to emerge during the long term, the authors extended the follow-up to five years, showing comparable outcomes with Nobori BES and CYPHER SES in terms of the composite primary endpoint (14.8% vs. 15.8%; OR 0.93, 95% CI: 0.75-1.16; p=0.53). Of note, this was the result of higher rates of the primary endpoint with the Nobori BES during the first year, compensated by lower rates beyond the first year up to five-year follow-up. Similar time-dependent changes in treatment effects were observed in other all-comer DES trials using the CYPHER SES as comparator, such as the SORT-OUT III6, PROTECT7, and LEADERS8 trials.
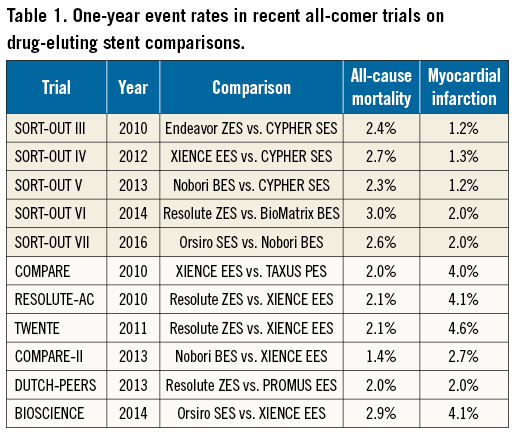
Jakobsen and co-authors should be congratulated for reporting the long-term follow-up of this important registry-based trial. Randomised trials based on national registries are cost-effective, pragmatic, and therefore of great value for the evaluation of medical devices. Notwithstanding this, registry-based randomised trials are subject to intrinsic potential inaccuracies related to discharge coding9,10 that may introduce a source of ascertainment bias. Specifically, adverse events with complex definitions (such as MI) or rare adverse events (such as ST) require an active screening and review of potential events by an independent clinical events committee for an adequate adjudication process. Conversely, the value of registry-based trials is undisputed for the evaluation of treatment effects on hard clinical endpoints of simple detection and definition such as all-cause mortality11. This is evident from the comparison of event rates between all-comer trials based on registries and those based on dedicated databases with active follow-up (Table 1). While rates of events easy to detect and adjudicate such as all-cause mortality are consistent across different trials, rates of MI appear to be affected by the study design.
The immediate clinical implications of the long-term findings of SORT-OUT V are limited, since the investigated DES are outdated. The manufacturing of the CYPHER SES was discontinued in 2011 and the Nobori BES has largely been replaced by newer biodegradable polymer-based DES. This reflects the rapid innovation in the field of coronary stents.
The observation of relative changes in device safety and efficacy over time, however, should influence the design of future studies investigating novel coronary devices such as fully bioresorbable scaffolds. It is noteworthy that contemporary new-generation DES have reduced the risk of ST to at least the level of bare metal stents, while maintaining or improving device effectiveness compared with early-generation DES12. In view of the low stent-related event rates occurring during the late follow-up phase with the use of contemporary DES, it appears unlikely that these may determine time-dependent changes in treatment effects as observed in SORT-OUT V.
Overall, the SORT-OUT V five-year findings highlight once again the need for a long-term evaluation of novel coronary devices in populations representative of routine clinical practice for a complete characterisation of their efficacy and safety profile. In this regard, a Task Force of the European Society of Cardiology (ESC)/European Association for Percutaneous Cardiovascular Interventions (EAPCI) has recently provided recommendations for a new regulatory process for coronary stents in Europe (Figure 1)1. While pointing out that the life cycle of coronary stents is short due to rapid device iteration and innovation – which can make devices clinically obsolete within five years – the Task Force acknowledged the critical role of long-term post-marketing surveillance in the clinical evaluation process of coronary devices. For this purpose, a close collaboration among device manufacturers, clinical investigators, physicians, and regulatory authorities is pivotal. However, the need for long-term follow-up should not delay but rather condition novel coronary device approval, since innovation remains the basis of therapeutic advances, and a timely access for patients to improved devices is warranted.
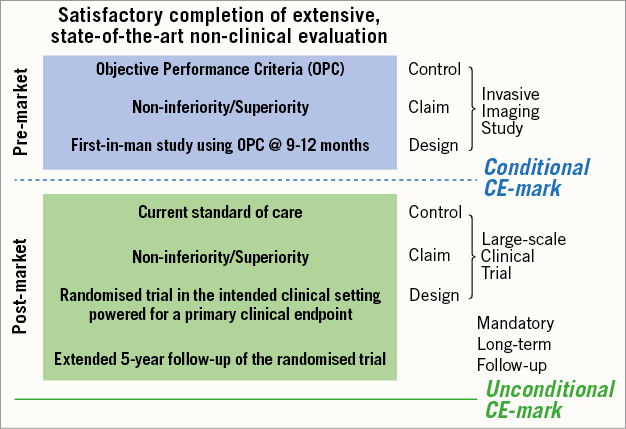
Figure 1. Clinical development plan proposed by the ESC/EAPCI Task Force on coronary stent evaluation. Adapted from Byrne et al. Eur Heart J. 20151 with permission.
Conflict of interest statement
G. Stefanini has received a research grant (to the institution) from Boston Scientific, and speaker/consulting fees from B. Braun, Biosensors, Boston Scientific, and Edwards Lifesciences. D. Cutlip has received research funding paid to the institution from Medtronic, Boston Scientific, and CeloNova BioSciences.

