
Introduction
The persistence of polymer material on first- and second-generation coronary drug-eluting stents after completion of drug release has been suggested as a trigger for a chronic inflammatory response1. Coronary drug-eluting stents with biodegradable polymers have been designed to overcome concerns over the delayed arterial healing, which might increase the risk of very late stent thrombosis and restenosis. The “Randomized Comparison of a Biodegradable Polymer Ultrathin Strut Sirolimus-Eluting Stent With a Biodegradable Polymer Biolimus-Eluting Stent in Patients Treated With Percutaneous Coronary Intervention: The SORT OUT VII Trial”2 demonstrated excellent one-year results with low target lesion failure rates. The present study extends the follow-up to two years.
Methods
OUTCOME MEASURES
The primary endpoint of target lesion failure is a composite of cardiac death, myocardial infarction (not related to other than index lesion) or clinically indicated target lesion revascularisation with percutaneous coronary intervention or coronary artery bypass operation within 24 months. The secondary endpoints were defined in the primary publication2.
STATISTICAL ANALYSIS
In analyses of every endpoint, follow-up continued until the date of an endpoint event, death, emigration, or 24 months after stent implantation, whichever came first. We constructed survival curves based on time to events, accounting for the competing risk of death (in cases of death not included in the outcome). Rate ratios (RR) were calculated for target lesion failure at 24-month follow-up. Rate ratios were calculated by modified Poisson regression analysis with a sandwich error estimation to assess whether difference detected at baseline had any effect on the result.
Results
A total of 2,525 patients were randomly assigned to receive either the biodegradable polymer sirolimus-eluting Orsiro stent (Biotronik, Bülach, Switzerland) or the biodegradable polymer biolimus-eluting Nobori® stent (Terumo Corp., Tokyo, Japan). Three patients were lost to follow-up (on days 6, 81 and 610) because of emigration. Complete follow-up data were available for 2,523 (99.9%) patients.
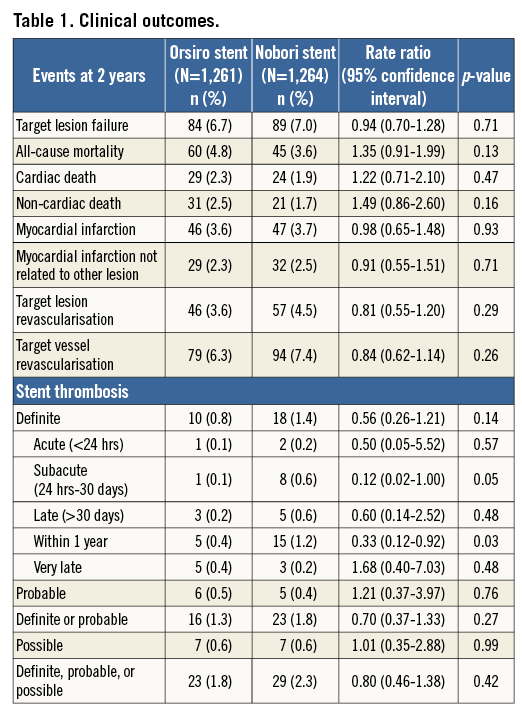
At two years, the composite endpoint target lesion failure had occurred in 84 patients (6.7%) in the biodegradable polymer sirolimus-eluting Orsiro group and in 89 patients (7.0%) in the biodegradable polymer biolimus-eluting Nobori group (RR 0.94, 95% CI: 0.70-1.28) (Figure 1, Table 1). Rates of death, cardiac death, myocardial infarction, and clinically driven target lesion revascularisation at two years did not differ significantly between the two stent groups. Adjusting for age and reference vessel diameter did not change the risk ratio significantly. At two years, the rate of definite stent thrombosis was numerically lower in the sirolimus-eluting Orsiro group (10 patients [0.8%] versus 18 patients [1.4%] in the biolimus-eluting Nobori group [RR 0.56, 95% CI: 0.26-1.21]). This reduced rate of definite stent thrombosis in the biodegradable polymer sirolimus-eluting stent was attributable to a lower risk of early definite stent thrombosis (Table 1). In contrast, the rate of very late stent thrombosis did not differ significantly between the two groups. Findings for the primary endpoint target lesion failure were consistent across pre-specified stratified analyses except for patients with multivessel disease and patients treated with more than one stent (Figure 2).
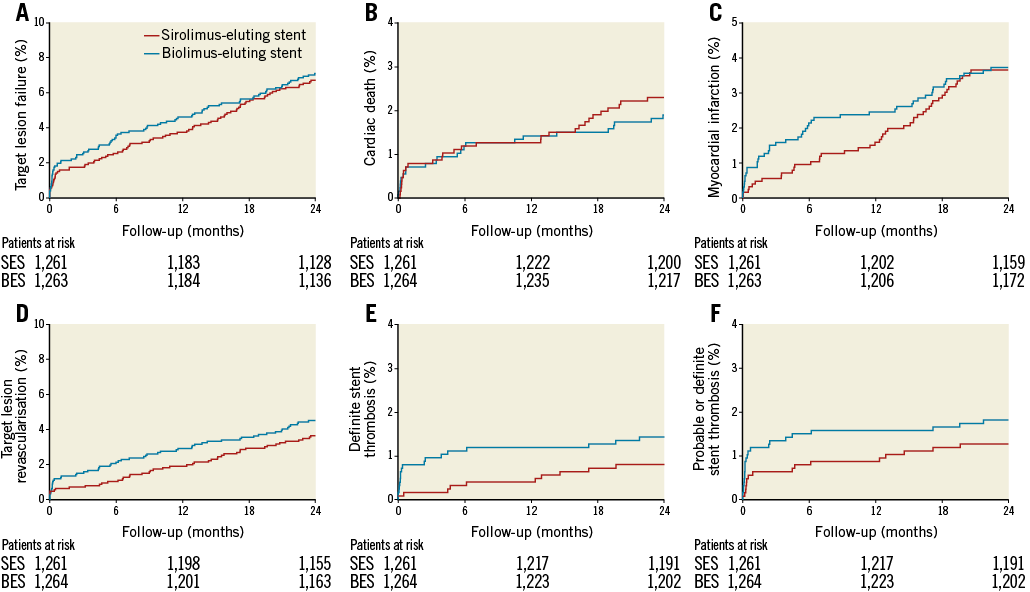
Figure 1. Time-to-event curves for major adverse cardiac events. A) Target lesion failure. B) Cardiac death. C) Myocardial infarction. D) Target lesion revascularisation. E) Definite stent thrombosis. F) Probable or definite stent thrombosis.
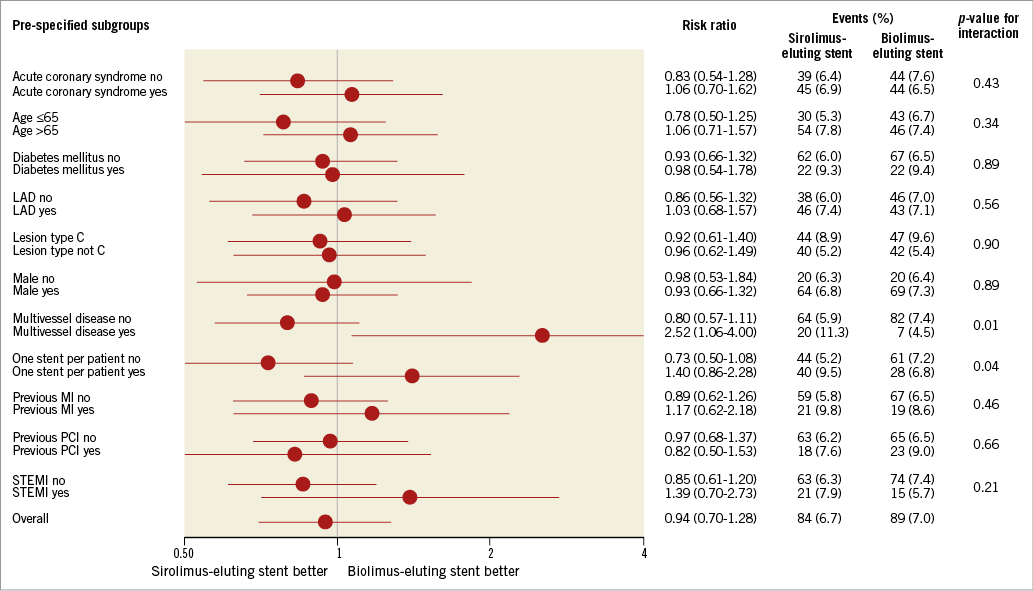
Figure 2. Pre-specified subgroup analysis for the primary endpoint at two-year follow-up.
Discussion
At one-year follow-up we documented non-inferiority of the sirolimus-eluting Orsiro stent and, across a variety of patient and lesion subgroups, the two treatments yielded a similar target lesion failure rate. This result was maintained at two years for the primary endpoint. Within the first year, the rate of definite stent thrombosis was lower in the sirolimus-eluting Orsiro stent group, which was attributable to a lower risk of stent thrombosis within 30 days. At two years, the number of instances of definite stent thrombosis was twice as high in the biolimus-eluting Nobori stent; however, the risk of very late stent thrombosis was similar in the two groups. This is in accordance with several other clinical trials, where the biolimus-eluting Nobori stent has been associated with an increased risk of early stent thrombosis, whereas the risk of very late definite stent thrombosis has been similar between the biolimus-eluting Nobori and the comparator stent3-5. The differences seemed to occur mainly during the first month and we cannot exclude that 1) slower drug release (12 weeks versus approximately 4 weeks), 2) slower polymer degradation (12-24 months compared to 6-9 months), 3) absence of a non-degradable parylene coating between the stent and the biodegradable polymer (as covering the entire Nobori stent), or 4) thinner stent struts (60-80 µm compared to the 120 µm), might be causal factors in reducing the inflammatory response and the risk of early stent thrombosis. In the “Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE)” trial, the biodegradable polymer sirolimus-eluting Orsiro was non-inferior to the durable fluoropolymer everolimus-eluting stent for target lesion failure at one year; no significant differences were noted in stent thrombosis. Comparable safety and efficacy profiles of the two stents were maintained throughout two years of follow-up6.
Limitations
The polymer degradation of the sirolimus-eluting Orsiro stent takes place after 12-24 months. Longer follow-up may show the influence of this polymer degradation.
Conclusions
Target lesion failure and the risk of very late definite stent thrombosis were similar for the biodegradable polymer sirolimus-eluting Orsiro stent and the biolimus-eluting Nobori stent in unselected patients at two years.
| Impact on daily practice In biodegradable polymer stents, the timing of polymer biodegradation and strut thickness may affect both vessel wall inflammation and late adverse outcomes following drug-eluting stent implantation. In an all-comer patient population, two-year target lesion failure was similar for the sirolimus-eluting Orsiro stent and the biolimus-eluting Nobori stent. |
Funding
This study was an investigator-initiated study and supported with equal unrestricted grants from Biotronik (Bülach, Switzerland), and Terumo (Tokyo, Japan). These companies did not have a role in study design, data collection, data analysis, or interpretation of results. Also, they did not have access to the clinical trial database or an opportunity to review the manuscript. The corresponding author had full access to all the data in the study and final responsibility to submit for publication.
Conflict of interest statement
L.O. Jensen has received research grants from Terumo, Biotronik, St. Jude Medical, and Biosensors to her institution. M. Maeng has received research grants from Boston Scientific, Biosensors International, and Volcano to his institution. The other authors have no conflicts of interest to declare.

