
Abstract
Aims: We aimed to assess the safety and performance of a novel sirolimus-eluting stent with biodegradable polymer under real-world conditions.
Methods and results: This prospective, multicentre, observational, all-comers registry enrolled 1,356 patients. The primary endpoint was target lesion failure at 12 months: it occurred in 5.1% (95% CI: 4.0-6.4) of patients in the overall population and in 7.7% (95% CI: 5.5-10.9), 5.8% (95% CI: 4.2-8.1), 1.8% (95% CI: 0.2-11.8) and 7.2% (95% CI: 5.1-10.0) of patients with diabetes mellitus, small vessels, chronic total occlusion and acute myocardial infarction, respectively.
Conclusions: This novel stent platform demonstrated good clinical outcomes in an all-comers population, even in predefined high-risk groups. ClinialTrials.gov identifier: NCT01553526
Introduction
Hybrid drug-eluting stents (DES) provide temporary drug exposure with subsequent degradation of the polymer, leaving a bare metal stent in the vessel, and hence address concerns associated with permanent polymer stents such as suboptimal polymer compatibility, delayed stent endothelialisation and late stent thrombosis1-3. The Orsiro (Biotronik AG, Bülach, Switzerland) hybrid DES has shown promising results in the BIOFLOW-I1 and BIOFLOW-II2 studies. We aimed to assess whether the good results of these selected patient populations can be replicated in unselected patients under real-world conditions and in predefined high-risk groups.
Methods and results
The BIOFLOW-III registry is a prospective, non-randomised, multicentre, observational all-comers registry to evaluate the safety and performance of the Orsiro DES in a large series of patients under real-world conditions with treatment according to standard of care and follow-up assessments up to 60 months. The Orsiro DES has been previously described1. In brief, it is made of a cobalt-chromium alloy which is covered by a thin layer of amorphous silicon carbide. The biodegradable polymer used as a carrier material for sirolimus is poly-L-lactic acid (PLLA).
The registry was conducted according to the Declaration of Helsinki and ISO 14155:2011 and approved by all relevant regional ethical review boards. All patients provided informed consent, 25% of randomly chosen subjects were monitored for endpoint-related data, and all serious adverse events were adjudicated by an independent clinical events committee. Data are presented using descriptive statistical methods. Predefined subgroups were compared using Fisher’s exact test, the chi-squared and the Student’s t-test. A Kaplan-Meier estimator, log-rank test and Cox proportional hazards model were used for survival analysis.
From August 2011 until March 2012, 1,356 patients with 1,738 lesions were enrolled at 43 sites in 14 countries (Figure 1). The baseline patient and lesion characteristics are shown in Table 1 and Table 2. Lesions were predominantly located in the left anterior descending artery (40%), followed by the right coronary artery (32%), the circumflex artery (22%), coronary artery bypass graft (CABG) (3%), left main (2%) and the anterolateral branch (1%). Predilatation was conducted in 65.9% of the treated lesions, and post-dilatation in 25.3%. Clinical device success and procedure success were achieved in 98.8% and 98.2% of the patients, respectively.
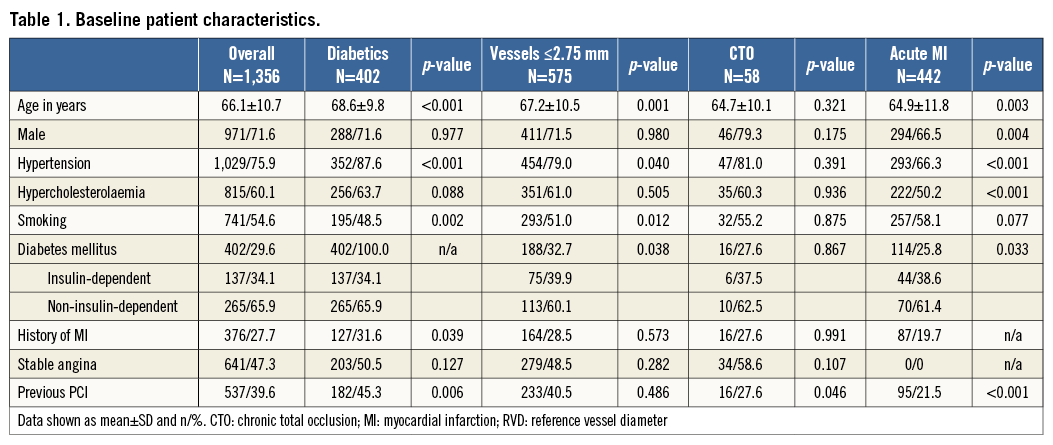
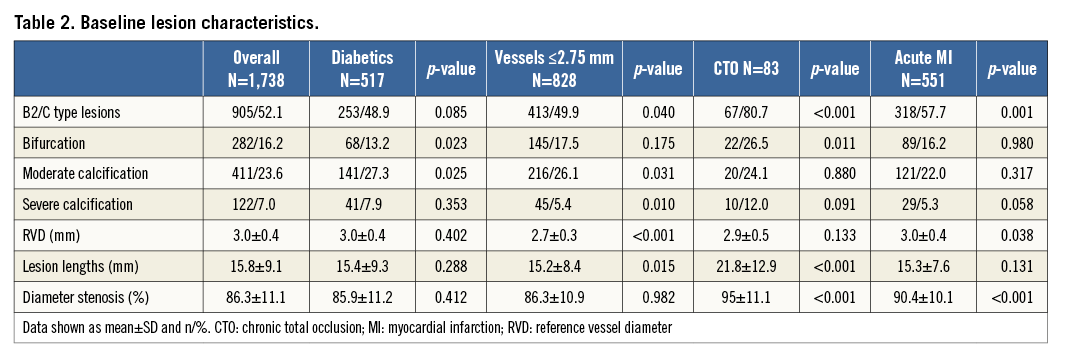
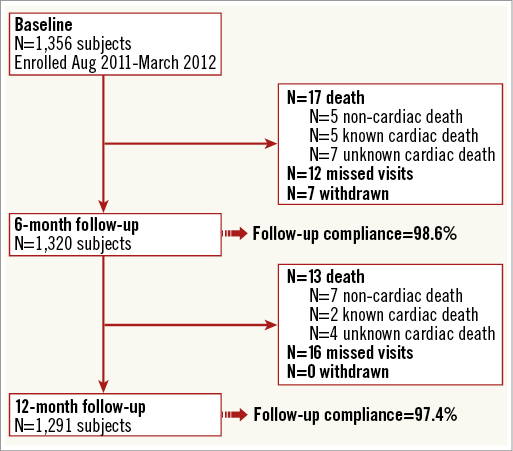
Figure 1. Flow chart of patients from baseline to 12-month follow-up.
The primary endpoint of the study, target lesion failure (TLF), a composite of cardiac death, target vessel myocardial infarction (MI), CABG and clinically driven target lesion revascularisation (TLR) at 12 months, was 5.1% (95% CI: 4.0-6.4) (Figure 2A). As displayed in Figure 3, diabetes mellitus, renal insufficiency and NSTEMI were associated with an increased TLF rate (HR 2.00 [95% CI: 1.23-3.23], HR 1.89 [95% CI: 1.03-3.46], and HR 2.09 [95% CI: 1.27-3.45], respectively). Kaplan-Meier estimates for the individual TLF components, target vessel revascularisation and definite stent thrombosis are provided in Figure 2B-Figure 2F.
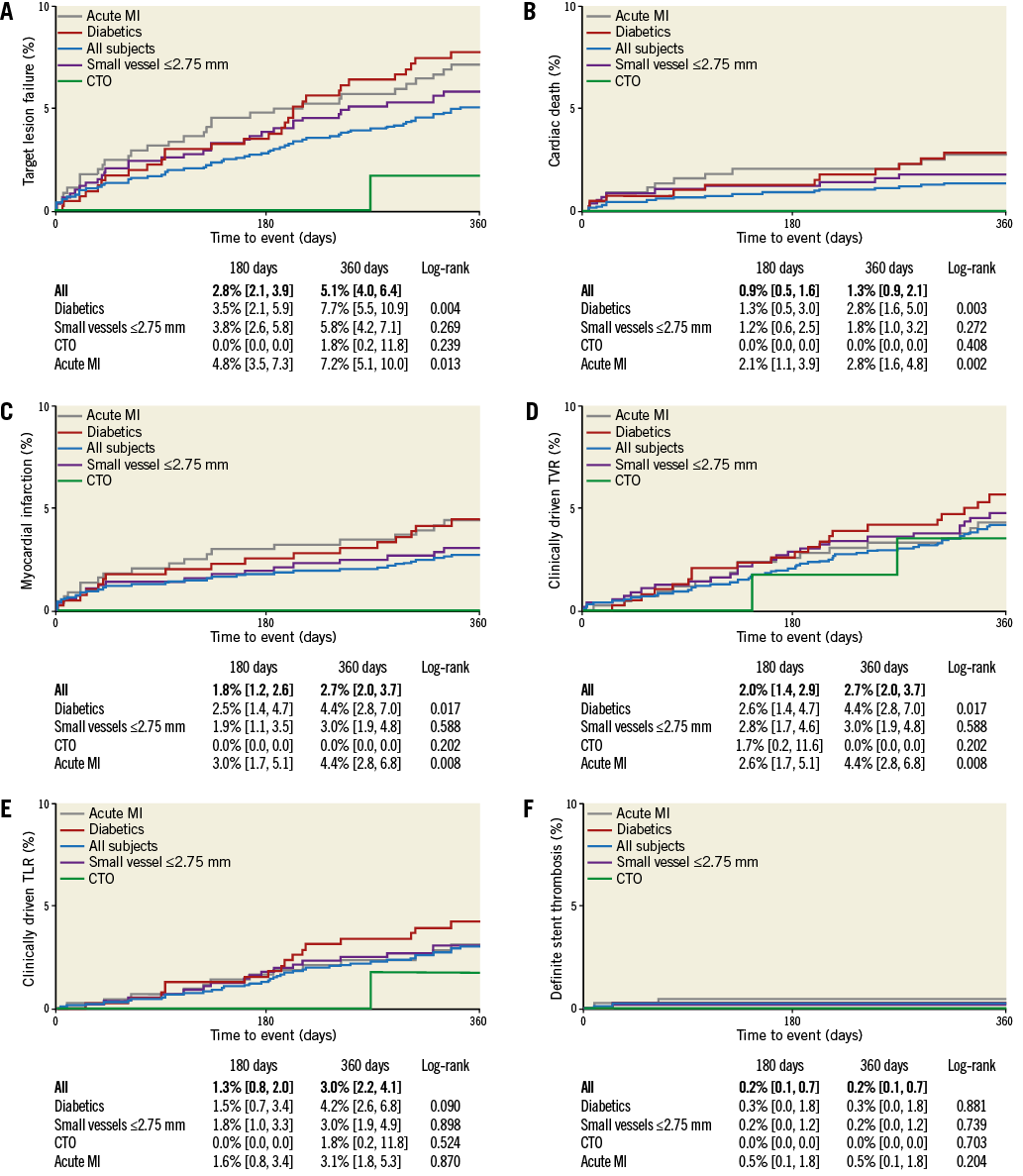
Figure 2. Kaplan-Meier event estimates. Kaplan-Meier event estimates for: A) target lesion failure, B) cardiac death, C) myocardial infarction, D) clinically driven target vessel revascularisation, E) clinically driven target lesion revascularisation, and F) definite stent thrombosis. CTO: chronic total occlusion; MI: myocardial infarction
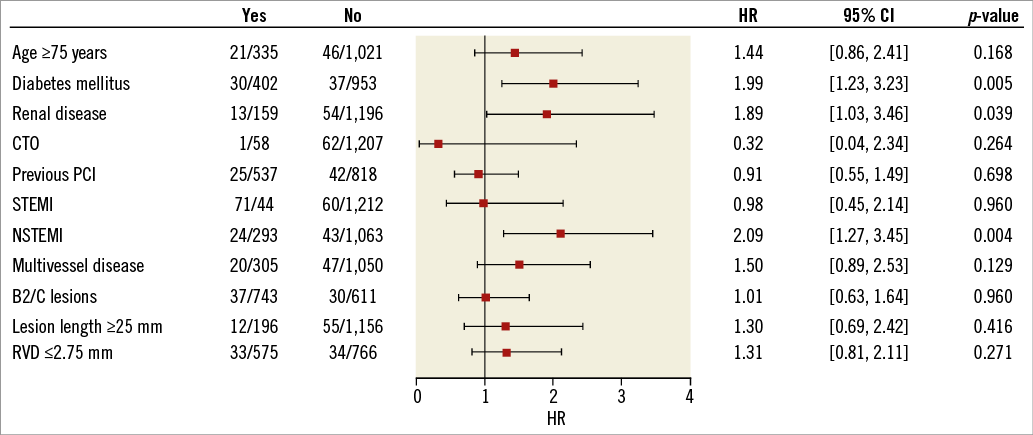
Figure 3. Cox proportional hazards for target lesion failure. Cox proportional hazards for target lesion failure with Wald test and Wald confidence limits for the hazard ratio. The squares indicate the hazard ratios and the horizontal lines indicate 95% confidence intervals. CTO: chronic total occlusion; HR: hazard ratio; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; RVD: reference vessel diameter; STEMI: ST-elevation myocardial infarction; TLF: target lesion failure
Discussion
Despite a high proportion of high-risk patients, the BIOFLOW-III registry had slightly better outcomes than those observed in the BIOFLOW-I1 and BIOFLOW-II2 studies (12-month composite of cardiac death, target vessel MI, CABG and TLR of 5.1% compared to 10.0%1 and 6.5%2). The outcomes of BIOFLOW-III also compare well with those of other biodegradable polymer stents such as BioMatrix™ (Biosensors Inc., Newport Beach, CA, USA) and Nobori® (Terumo Corporation, Tokyo, Japan). At 12 months, cardiac death was 1.3% for Orsiro compared to 0.8%-1.2% for Nobori3-5 and 2.1% for BioMatrix6, MI was 2.7% versus 1.5%-2.8%3-5 and 5.9%6, clinically driven TLR was 3.0% compared to 2.2%-3.3%3-5 and 6.0%6, and definite stent thrombosis was 0.2% compared to 0.7%3,5 and 2.0%6. Furthermore, the data compare well with the outcomes of a recent meta-analysis including 60 trials with DES7.
In the predefined groups, patients with diabetes and patients with acute MI had significantly higher TLF, cardiac death and MI rates. There was no such difference for patients with small vessels. Correspondingly, Cox proportional hazards for TLF identified diabetes mellitus, renal disease and NSTEMI as influencing factors.
To the best of our knowledge, BIOFLOW-III is the first trial to assess clinical outcomes of biodegradable polymer stents in patients with CTO. The TLF rate of 1.8% at 12 months was good and was attributed to TLR only. However, only 58 patients were enrolled in the CTO group, hence results have to be interpreted with caution and would need to be confirmed in a larger series of patients. Notably, the registry was not powered to detect differences in subgroups, hence the results of the subgroup analysis must be regarded as hypothesis-generating only.
Conclusions
The low 12-month TLF rate of 5.1% and definite stent thrombosis rate of 0.2% in this all-comers setting imply safety and effectiveness of the Orsiro DES with biodegradable polymer. Low event rates were also confirmed in subgroups of diabetics, small vessels, CTO and acute MI.
| Impact on daily practice Drug-eluting stents with biodegradable polymer are novel devices which should address concerns asociated with permanent polymer stents. BIOFLOW-III shows, in a large patient population of more than 1,300 patients, that a novel DES with biodegradable polymer can be safely applied in an all-comer population, even in high-risk populations such as those with diabetes mellitus, small vessels, chronic total occlusions and acute myocardial infarction. |
Funding
The study was supported by Biotronik AG, Bülach, Switzerland.
Conflict of interest statement
J. Waltenberger and A. Erglis have received lecture honoraria and consulting fees from Biotronik. O. Fröbert has received consulting fees, G. Richardt and S. Hofmann have received lecture honoraria, and M. Winkens has received research grants from Biotronik. The other authors have no conflicts of interest to declare.

