Abstract
Aims: Drug-eluting stents (DES) are a major advance in interventional cardiology; however concerns have been raised regarding their long-term safety due to the permanent nature of the polymer. New generation stents with biodegradable polymers (BDS) have recently been developed. The aim of this study was to perform a meta-analysis of randomised controlled trials (RCTs) comparing the safety and efficacy profile of BDS vs. durable polymer DES.
Methods and results: The MEDLINE/CENTRAL and Google Scholar databases were searched for RCTs comparing safety and efficacy of BDS vs. DES. Safety endpoints were mortality, myocardial infarction (MI), and stent thrombosis (ST). Efficacy endpoints were target vessel revascularisation (TVR), target lesion revascularisation (TLR) and six-month in-stent late loss (ISLL). The meta-analysis included eight RCTs (n=7,481). At a median follow-up of nine months, as compared to DES, BDS use did not increase mortality (OR [95% CI] = 0.91 [0.69-1.22], p=0.53) or MI (OR [95% CI] = 1.14 [0.90-1.44], p=0.29). Rate of late/very late ST was significantly reduced in BDS patients (OR [95% CI] = 0.60 [0.39-0.91], p=0.02), as was six-month ISLL (mean difference [95% CI] = –0.07 [–0.12; –0.02] mm, p=0.004) in comparison with DES patients. Rates of TVR and TLR were comparable between BDS and DES.
Conclusions: BDS are at least as safe as standard DES with regard to survival and MI, and more effective in reducing late ST, as well as six-month ISLL. Further large RCTs with long-term follow-up are warranted to definitively confirm the potential benefits of BDS.
Abbreviations
BDS: biodegradable polymer drug-eluting stents
CI: confidence interval
DES: drug-eluting stents
ISLL: in-stent late loss
MACE: major adverse cardiac event
MD: mean difference
MI: myocardial infarction
OR: odds ratio
RCTs: randomised controlled trials
SE: standard error
ST: stent thrombosis
TLR: target lesion revascularisation
TVR: target vessel revascularisation
uLM: unprotected left main disease
Introduction
Drug-eluting stents (DES) are a major advance in interventional cardiology owing to their ability to reduce restenosis and related target vessel revascularisation (TVR), mainly by limiting intimal hyperplasia1,2. However, concerns regarding the long-term safety of DES using permanent polymer technology are increasing3. Recent studies showed that the durable presence of the polymer coating may contribute to stent thrombosis (ST) and to a late catch-up phenomenon (delayed restenosis), as a consequence of delayed healing and hypersensitive reaction4,5. These findings have prompted efforts to develop alternative stents with biocompatible and biodegradable polymers (BDS) for drug delivery. The aim of the present meta-analysis is to compare the safety and efficacy profile of BDS vs. DES.
Methods
Established methods6 were used in compliance with the PRISMA statement for reporting systematic reviews and meta-analyses in health care interventions7.
SEARCH STRATEGY
A search covering the period from November 1994 to November 2010 was conducted by two independent investigators using MEDLINE/CENTRAL and Google Scholar databases, and conference proceedings from the American College of Cardiology, American Heart Association, European Society of Cardiology, Transcatheter Cardiovascular Therapeutics and EuroPCR scientific sessions. The following keywords were applied: “coronary biodegradable polymer”, “drug-eluting stent”, “biolimus stent” and “coronary angioplasty”. References of retrieved studies were searched manually for additional trials, and efforts to contact authors or manufacturers of BDS were performed to obtain further details or additional references. No language restrictions were applied. Data were abstracted on pre-specified forms by two independent investigators, neither involved in any of the studies retrieved; divergences were resolved by discussion with a third investigator.
SELECTION CRITERIA
Citations were screened at title/abstract level and retrieved as full reports. Inclusion criteria were: 1. human studies; 2. randomised controlled studies (RCTs); 3. studies comparing safety and/or efficacy of BDS vs. DES; 4. additional data from retrieved studies available at a longer follow-up. Exclusion criteria were: 1. non-RCTs; 2. sub-study of the RCT; 3. bare metal stent (BMS) as control group. Internal validity was appraised by two unblinded investigators according to proper allocation sequence/concealment, patient blinding, investigator blinding and complete outcome data/full reporting.
STUDY ENDPOINTS
The primary safety endpoint was all-cause mortality. Secondary safety endpoints were myocardial infarction (MI), any ST according to the Academic Research Consortium (ARC) classification8. Efficacy endpoints included any TVR, any target lesion revascularisation (TLR) and six month in-stent late loss (ISLL). The follow-up data available from the initial published studies were analysed. Furthermore, new data with extended follow-up that became available through a second publication or during congress presentations were additionally analysed for mortality, TLR, TVR, and late/very late ST outcomes.
STATISTICAL ANALYSIS
Data were analysed according to the intention-to-treat principle. Odds ratio (OR) and 95% confidence intervals (95% CI) were used as summary statistics. Heterogeneity was assessed by Cochran’s Q test, with a 2-tailed p=0.19. The statistical inconsistency test (I2) {[(Q-df)/Q]×100%, where Q is the chi-squared statistic and df its degrees of freedom} was also employed to overcome the low statistical power of Cochran’s Q test10. Pooled ORs were calculated using a fixed effect model with the Mantel-Haenszel method. The DerSimonian and Laird random effects model was used in case of significant heterogeneity and/or moderate or significant inconsistency (>50%) across studies6. For the ISLL outcome, the mean difference (MD) of six-month ISLL compared with baseline was used. The overall weighted MD was built with the inverse variance method. Potential publication bias was examined by constructing a “funnel plot”, in which the standard error (SE) of the ln OR or MD was plotted against the OR (for mortality, MI, ST, major adverse cardiac event [MACE], TVR, TLR) or the MD (for six-month ISLL ). A mathematical estimate of the asymmetry of this plot was provided by a linear regression approach11,12. Finally, we addressed the influence of each study by testing whether, deleting each in turn, would have changed significantly the pooled results of the meta-analysis (sensitivity analysis). Review Manager 5.1 (The Nordic Cochrane Center, Købehvn, Denmark) and Comprehensive Meta-Analysis version 2 (Biostat, Englewood, NJ, USA) were used for statistical computations.
Results
LITERATURE SEARCH
A PRISMA flow chart, describing the process of publication screening and the reasons for exclusion, is shown in Figure 1. From 450 initial references, 434 were excluded during preliminary screening. The remaining 16 articles were scrutinised for eligibility. Eight RCTs fulfilling the eligibility criteria were finally included for data abstraction13-20. Seven of these were full text articles13-18,20, the remaining one was available as an abstract with additional data slides presented during medical congresses. Additional two- or three-year follow-up data on mortality, MI, late/very late ST, MACE, TVR, and TLR were available for ISAR TEST-321, LEADERS (2010 Transcatheter Cardiovascular Therapeutics Congress), ISAR TEST-4 (2010 European Society of Cardiology Congress) and NOBORI I, phase 1 and 2 combined (2010 EuroPCR Congress).
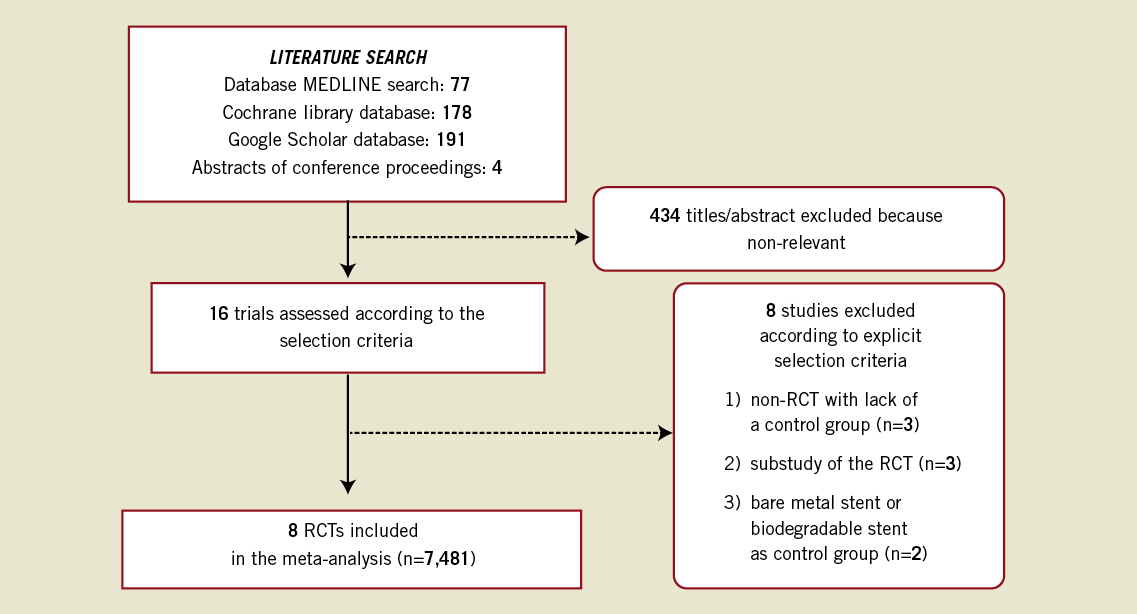
Figure 1. Flow diagram of the review process according to the PRISMA statement.
ELIGIBLE STUDIES
The eight RCTs included in the meta-analysis involved 7,481 patients, with 3,985 and 3,496 allocated to the BDS and DES, respectively.
In all the studies MI was defined (except for NOBORI JAPAN, which did not report the definition) as either development of pathological Q-waves in at least two contiguous leads with or without elevated cardiac enzymes, or in absence of pathological Q-waves, as an elevation in creatine kinase levels to greater than twice or at the upper limit of normal in presence of an elevated level of CK-MB fraction.
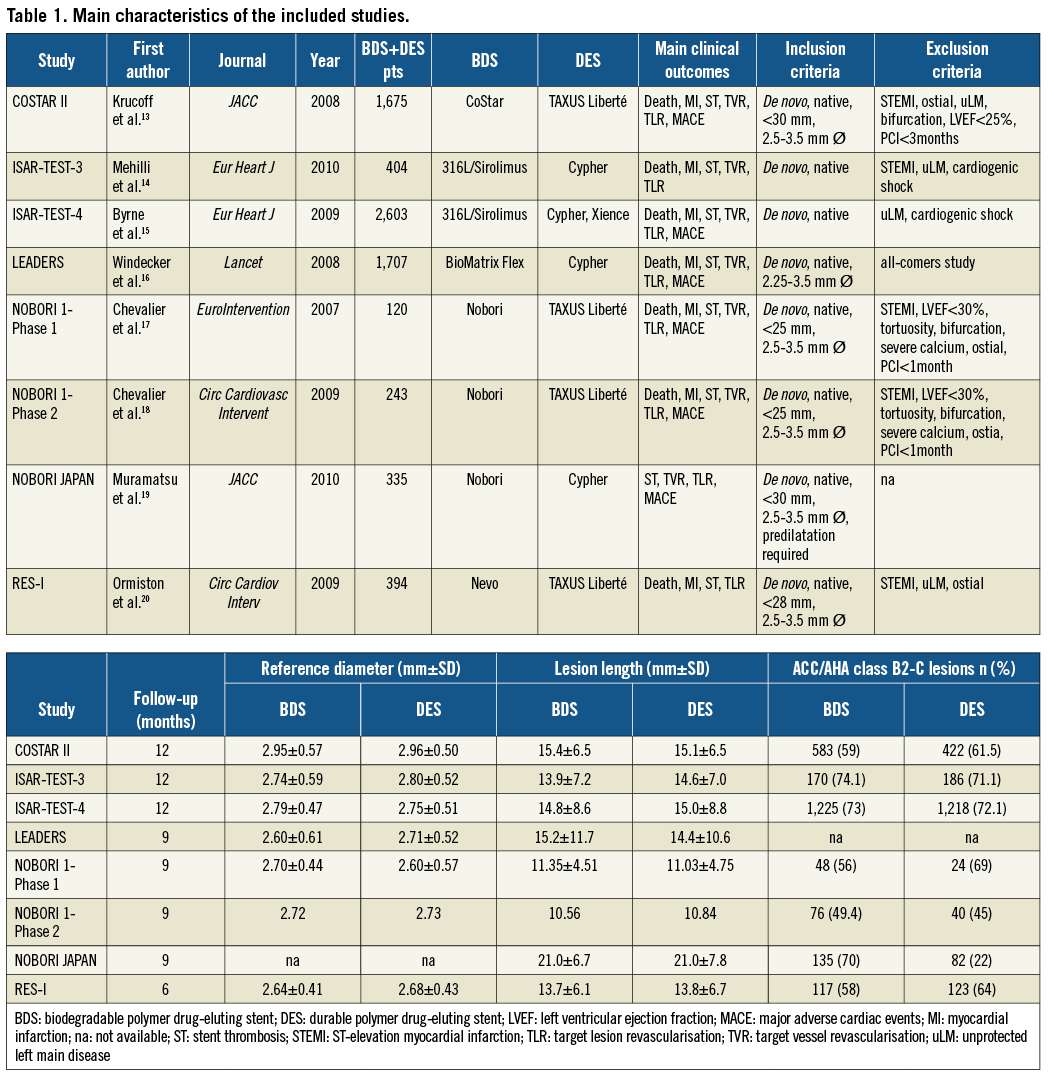
The definition used for TVR or TLR was any target vessel or target lesion revascularisation where stenosis of the treated lesions was at least 50% and prompted by a positive functional study or by ischaemic ECG changes at rest in a distribution consistent with the target vessel or by ischaemic symptoms. The definition was not available for NOBORI JAPAN. Table 1 lists the main study characteristics. Median follow-up was nine months. The main exclusion criteria (except for the LEADERS trial, which was an all-comer study) were ST-elevation MI, bifurcation lesions, ostial lesion location, and unprotected left main disease. Among the treated lesions, 2,354 (71%) in the BDS group vs. 2,095 (63%) in the DES group were of class B2-C according to the ACC/AHA classification; mean lesion length was 13.55 mm in the BDS group as compared to 13.53 mm in the DES group. Mean age was similar in the two groups. Men represented 74.5% of the BDS and 74.7% of the DES population. There were 1,083 patients with diabetes in the BDS group (27.2%) and 944 in the DES group (27%).
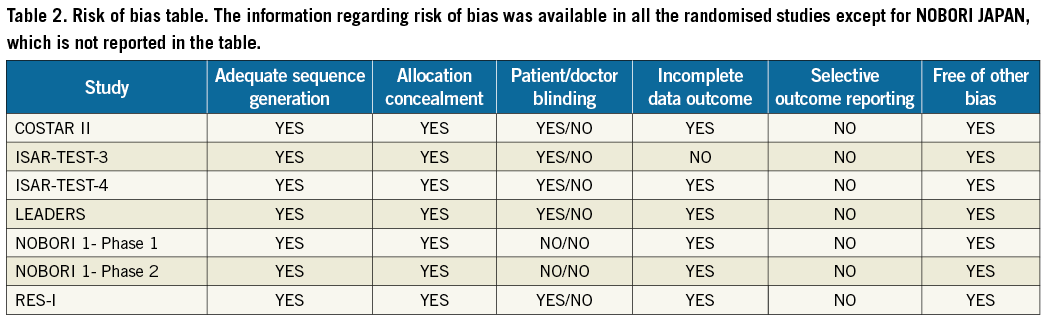
Table 2 illustrates a risk of bias in analysis in which studies are judged by a set of six criteria defined by the Cochrane Collaboration (http://www.cochrane.org/). Outcomes were adjudicated by blinded central committees in three trials (ISAR TEST-3, ISAR TEST-4, LEADERS). The Nobori stent was the most used BDS (50% of the patients in the BDS arm); with regard to the DES arm, all the patients from the included studies were treated with first generation DES, sirolimus or paclitaxel, except for those from the ISAR-TEST-4 trial (half of whom received an everolimus-eluting stent).
Safety endpoints
MORTALITY
The funnel plot for mortality did not show asymmetry on visual inspection (Figure 2) and this was confirmed by Egger’s test that was not significant (p=0.21). The forest plot showing the OR for mortality for the eight studies included (involving a total of 7,464 patients) is shown in Figure 3A. The median follow-up time was nine months. There were a total of 195 deaths. The incidence of death was 2.3.% (95/3,977) in the BDS group and 2.8 % (100/3,487) in the DES group (OR [95% CI] = 0.91 [0.69-1.22], p=0.53, p for heterogeneity=0.88).
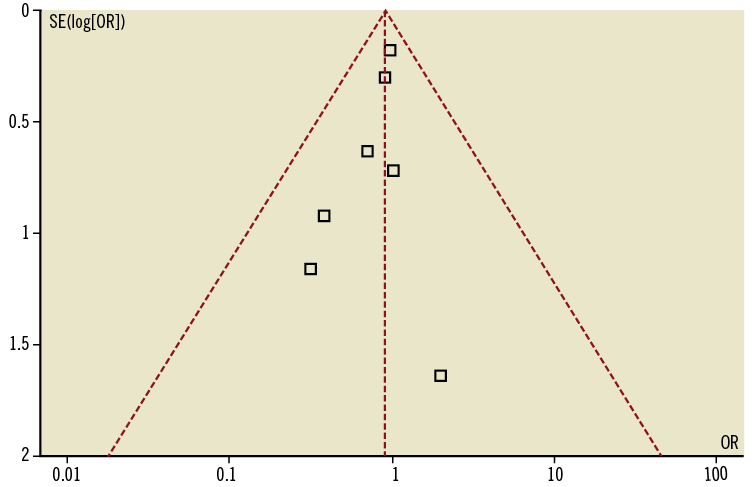
Figure 2. Funnel plot for mortality outcome. The sample size of each study (measured as standard error of the treatment effect) was plotted against the odds ratio for overall mortality.
ADDITION OF LONGER FOLLOW-UP DATA ON MORTALITY
The longest median follow-up was 18 months. The pooled estimate after adding the new follow-up data from ISAR-TEST-3, LEADERS and NOBORI I, phase 1 and 2 combined did not deviate from the previous one (OR [95% CI] = 0.87 [0.67-1.13], p=0.29, p for heterogeneity= 0.92).
MYOCARDIAL INFARCTION
By visual inspection of the funnel plot for MI and by Egger’s test (p=0.1) there was no evidence of publication bias. Among 7,464 patients, there were 291 MI. There was no significant difference in the rate of MI with BDS as compared with DES at a nine-month follow-up: 4.0% (163 of 3,977 patients) in the BDS group and 3.6% (128 of 3,487) in the DES group (OR [95% CI]=1.14 [0.90-1.44], p=0.29, p for heterogeneity = 0.79) (Figure 3B).

Figure 3. A. Individual and summary odds ratios for mortality in patients treated with BDS vs. DES; B. Individual and summary odds ratios for MI in patients treated with BDS vs. DES.
ADDITION OF LONGER FOLLOW-UP DATA ON MI
At a median follow-up of 18 months the results for MI confirmed the previous ones (OR [95% CI]=OR [95% CI]=1.05 [0.83-1.32], p=0.69, p for heterogeneity=0.68).
STENT THROMBOSIS
Any stent thrombosis was used as endpoint in seven of the eight RCTs, including “definite/probable” and “possible” when available, according to the ARC classification8; COSTAR II, instead, used a per protocol definition. ST was investigated at short-term follow-up. No publication bias was found by visually inspecting the funnel plot and by Egger’s test (p=0.4). A pre-specified stratified analysis for early and late/very late ST (≥one month) was also performed. There were 114 overall ST among 7,481 participants (1.5%) at a median follow-up of nine months. Rates were slightly but not significantly inferior in patients treated with BDS (1.3%) as compared to patients receiving DES (1.7%) (OR [95% CI] = 0.83 [0.58-1.21], p=0.34, p for heterogeneity=0.34) (Figure 4A).

Figure 4. A. Individual and summary odds ratios for stent thrombosis in patients treated with BDS vs. DES. B. Individual and summary odds ratios stratified for early and late/very late stent thrombosis.
LATE/VERY LATE ST
Data from LEADERS, NOBORI I phase 1 and 2 , ISAR-TEST-3 and ISAR TEST-4 at longest follow-up were used in the stratified analysis for late/very late ST. At a median follow-up of 24 months the meta-analysis showed a significant benefit for BDS, with the same direction of effect in all included studies (OR [95% CI]=0.60 [0.39-0.91], p=0.02, p for heterogeneity=0.55) (Figure 4B).
Efficacy endpoints
TARGET VESSEL REVASCULARISATION
All of the included studies reported any TVR, except for COSTAR II, which used clinically-driven TVR as outcome. There was no suggestion of publication bias by the funnel plot and by Egger’s test (p=0.9). TVR was reported in 616 out of 7,097 patients (8.7%). Median follow-up was nine months. As shown in Figure 5A, the rate of TVR did not differ between the two groups (9.0% vs. 9.0%; OR [95% CI] = 0.98 [0.68-1.42], p=0.93, p for heterogeneity= 0.02).

Figure 5. A. Individual and summary odds ratios for target vessel revascularisation in patients previously treated with BDS vs. DES. B. Individual and summary odds ratios for target lesion revascularisation in patients previously treated with BDS vs. DES.
ADDITION OF LONGER FOLLOW-UP DATA ON TVR
The median follow-up was 18 months. Similar results to the previous ones were obtained for TVR rates at longer follow-up (OR [95% CI]=1.06 [0.70-1.60], p=0.78 [random effect model], p for heterogeneity=0.03).
TARGET LESION REVASCULARISATION
Rates of any TLR were available in seven studies. Asymmetry in the funnel plot was confirmed by Egger’s test (p=0.003). After exploring potential bias using the Duval and Tweedie trim and fill method, the observed estimates were comparable to the adjusted estimates, suggesting valid estimates with trivial publication bias effect. Median follow-up was nine months. Per treatment, TLR was 5.9 % (177/2,996) for BDS and 7.2% (202/2,810) for DES. Figure 5B shows the number of TLR according to treatment, with the OR for each trial. The overall OR was 0.84 with 95% CI 0.68-1.03, p=0.10, p for heterogeneity=0.24.
ADDITION OF LONGER-TERM FOLLOW-UP DATA ON TLR
Median follow-up was 18 months. The initial results were confirmed with an OR (95% CI)=0.86 (0.71-1.03), p=0.09, p for heterogeneity=0.39.
SIX-MONTH IN-STENT LATE LOSS
Six studies reported mean ISLL and SD for the two arms (BDS vs. DES) (Figure 6). The COSTAR trial did not report standard deviations, which are crucial to assign a statistical weight in the meta-analysis, so it was excluded from the computations. Follow-up was six months. NOBORI I (phase 2) reported nine-month follow-up. Mean ISLL in the BDS group was 0.15 mm vs. 0.24 mm in the DES group. Patients treated with BDS had significantly less ISLL than patients receiving DES (MD [95% CI] = –0.07 [–0.12; –0.02] mm, p=0.004 [random effect model], p for heterogeneity=0.02).

Figure 6. Individual means and standard deviations for six-month in-stent late loss and overall weighted mean difference in patients treated with BDS vs. DES.
SENSITIVITY AND SUBGROUP ANALYSES
Sensitivity analysis, performed by removing each of the studies one at a time, did not demonstrate any single study influencing the overall results for both the safety and the efficacy endpoints. Stratified analysis for the specific type of first generation DES (paclitaxel or sirolimus) confirmed the results of the overall analysis on mortality, as the primary endpoint (p interaction=0.19) and on the other selected endpoints; the direction of the effect on ST was maintained in favour of BDS when paclitaxel or sirolimus DES were used as the control group, with a non-significant trend in less late ST when BDS were compared with the paclitaxel stent (p interaction=0.07). With regard to ISLL, the overall results in favour of BDS were confirmed when analysed separately for the specific DES type used as control group (sirolimus or paclitaxel): a. MD (95% CI)=–0.04 (–0.07; –0.01) mm, p=0.006 (sirolimus); b. MD (95% CI)=–0.19 (–0.28; –0.11) mm, p<0.001 (paclitaxel). Stratified analysis for the BDS subtype (Nobori or others) was also carried out and confirmed the results obtained in the overall analysis for all the outcomes chosen. In addition to the fixed effect model, a random effects model was used for late stent thrombosis and confirmed the direction of the results in favour of BDS (OR [95% CI]=0.63 [0.41-0.97], p=0.04).
Discussion
This study is the first meta-analysis of RCTs comparing the safety and efficacy profile of BDS vs. DES use among 7,481 patients. The main finding is that patients allocated to BDS showed survival rates comparable to those treated with DES, with significantly less late/very late ST and six-month ISLL.
DES releasing sirolimus or paclitaxel from durable polymers have been shown to reduce angiographic and clinical measures of restenosis compared with bare-metal stents1,2. However, first generation DES have also been associated with higher rates of very late ST and of restenosis, attributed to delayed healing and delayed re-endothelisation due to the presence of the durable polymer coating4,5. Since permanent polymer coatings may have pro-inflammatory and thrombogenic potential, current DES research has focused on the use of biodegradable polymer coatings, which offer the attractive prospect of controlled drug-release without the potential of late polymer-associated adverse effects22,23.
There are in vitro data that raise issues with regard to biodegradable polymer technology: 1. It has been demonstrated that the polymer-based coating of the biodegradable stent (Biomatrix, Biosensors, Singapore) provides lower elasticity than durable polymers, which may lead to defects and fragility (cracks) of the coating following stent expansion during more than mild overstretch of the stent24; therefore, after post-dilatation, embolisation of material and microvascular obstruction could occur or there might be reduced antiproliferative power because of detachment of polymer fragments; 2. The chronic swelling of the stent as it absorbs water to dissolve has been shown to influence the degree of neointimal hyperplasia25. However, because the biodegradable polymer is expected to be totally degraded within 12 months following device implantation, the stent irregularities are unlikely to result in unfavourable clinical events; these data therefore are only hypothesis-generating and need to be confirmed in large clinical trials with a long follow-up. Several recently-published or presented clinical RCTs have performed head-to-head comparisons of the first generation DES with the new BDS.
The LEADERS16 study with an all-comer design was the first head-to-head comparison of a stent platform eluting biolimus from a biodegradable polymer with a first generation sirolimus-eluting stent (SES). At nine-months follow-up, the primary endpoint, a composite of death, MI and TVR, occurred in 9.2% of patients treated with BDS and 10.5% of patients treated with DES, demonstrating the non-inferiority of BDS compared to the DES (p for non-inferiority=0.003). Similarly, the ISAR-TEST-415 with 2,600 enrolled patients compared a novel biodegradable polymer-based, rapamycin-eluting stent with the two leading limus-based DES, the Cypher (using sirolimus) (Cordis, Johnson & Johnson, Warren, NJ, USA) and the Xience (using everolimus) (Abbott Laboratories, Abbott Park, IL, USA). At both 30 days and 12 months, BDS were significantly non-inferior (p=0.005) to the limus DES for a composite endpoint that included both safety (cardiac death/MI) and clinical restenosis (TLR). Conversely, the COSTAR DES trial13 found that the novel platform was not non-inferior to a Taxus DES (Boston Scientific, Natick, MA, USA). At eight months, the incidence of MACE (11.0% vs. 6.9%, p<0.005) and late loss (0.49 mm vs. 0.18 mm, p <0.0001) were significantly higher with the COSTAR stent.
This is the first meta-analysis that addresses the comparison between BDS and DES and collects evidence in the literature of all published and unpublished randomised trials, with a total of 7,481 enrolled patients. The present meta-analysis supports the safety of BDS vs. DES with comparable rates of mortality and MI; a significant reduction in any late stent thrombosis with BDS (0.91 %) as compared with DES (1.68%) was also observed; this finding may be attributable to long-term thrombogenic effects due to the persistence of the DES-associated durable polymer; it is conceivable that the result of a reduced rate of late ST might in turn translate into less deaths and acute coronary syndromes with the availability of longer follow-up data and an increased number of clinical event rates; in fact, when looking at the trend of survival curves in the included studies, it appears that with increasing follow-up there is an increasingly lower mortality or MACE with BDS as compared with DES, especially in patients with high risk profile and a SYNTAX score >16 ( long-term follow-up data from LEADERS): delta for mortality 4.3% at one year vs. 5.8% at three years in favour of BDS; high risk patients with complex lesions are a category which is often under-represented in RCTs, where the randomised population could be far from representative of real world population treated with the devices.
This meta-analysis did not show a clear advantage for BDS, as compared to DES, in the rates of TLR and TVR, despite a significant six-month reduction in ISLL observed among the BDS-treated patients. ISLL is a frequently used parameter to quantify the degree of neointimal hyperplasia after coronary stenting26. A strong, direct and significant association between ISLL and clinical impact, measured as number needed to treat to prevent one TLR, has also been demonstrated27. The lack of association between ISLL and TLR in the current meta-analysis may be explained by the fact that the majority of the included RCTs were underpowered for low rates of binary events, such as TLR, while the use of a continuous variable like ISLL allowed the efficacy of BDS vs. DES to be compared without the need for extremely large patient populations. The finding of a decreased ISLL with BDS as compared to DES in our meta-analysis suggests a potential anti-restenotic efficacy of BDS; however, caution must be recommended in interpreting this result, which needs to be confirmed in future large RCTs with longer follow-up.
Limitations
The majority of patients from the included studies received first generation DES, sirolimus or paclitaxel, except for one study (ISAR-TEST-4) in which half of the patients in the DES arm also received a new generation DES (everolimus); as a result, future RCTs and meta-analyses are certainly needed to confirm our findings for BDS compared with second generation DES (zotarolimus or everolimus).
As is well known for meta-analyses, the availability of individual patient data would have added further value to the results of the current study; differences in devices, platform and drugs, and inclusion criteria could have affected to some extent the results, so that caution is recommended in interpreting the results; however, there are several arguments that support the reliability of the results: 1. There was no statistical heterogeneity in the results for mortality and late ST; 2. The same direction of effect was observed for all the included studies when a significant result was found and all the trials fell to the left of the line of unity (favouring BDS); 3. Sensitivity and subgroup analyses showed that results were in compliance with the direction and magnitude of the effect found in the overall analysis.
Finally, very little information was available from the various RCTs on the subsets of patients with complex lesions (e.g., bifurcations), ST-elevation myocardial infarction, or diabetes that warrant further investigations.
Conclusions
BDS, as compared with DES, showed a good safety profile with no suggestion of increased mortality and a reduced incidence of late/very late ST. The rates of six-month ISLL were also significantly lower in BDS-treated patients in comparison with DES-treated ones.
Acknowledgements
EP Navarese is the recipient of a fellowship grant in interventional cardiology by the European Association of Percutaneous Cardiovascular Interventions (EAPCI).
Conflict of interest statement
The authors report no conflicts of interest to disclose.

