
Percutaneous coronary intervention with drug-eluting stents (DES) has substantially improved clinical outcomes in patients with coronary artery disease, when compared with bare metal stents. However, permanent polymers of first-generation DES were linked with local inflammation, hypersensitivity reactions, and neoatherosclerosis, leading to an increased risk of very late stent thrombosis and DES failure at long-term follow-up. Recently, studies and meta-analyses have shown superiority of the new-generation DES with biodegradable or biocompatible polymer to previous-generation DES in terms of improved clinical outcomes1,2. However, whether the long-term clinical performance of these two types of DES after implantation is comparable remains debatable.
The type of polymer coating remains a “hotly” debated topic in DES design and it may have a significant impact on clinical safety and efficacy in patients undergoing DES implantation. A pooled analysis of the ISAR-TEST 3, ISAR-TEST 4 and LEADERS trials showed that biodegradable polymer DES significantly reduced very late definite stent thrombosis compared with first-generation DES at long-term follow-up3. Of note, network meta-analyses comparing contemporary stents with a greater statistical power showed that biodegradable polymer DES were associated with an increased risk of early stent thrombosis and myocardial infarction compared with durable compatible polymer everolimus-eluting stents (EES)4. Therefore, the concept of fully biodegradable polymer coating technologies has been challenged. However, one should be very careful to interpret the findings of these indirect comparisons.
First, it is inappropriate to mix all the biodegradable polymer stents together, as some of them are known to be associated with significantly higher rates of adverse events. Second, despite including >90,000 patients in >100 trials, variable study protocols with different baseline demographics and clinical presentations provided poor comparison between the study devices. Third, very limited long-term follow-up data after biodegradable polymer DES implantation have been reported. Finally, caution is warranted in making firm conclusions from the Bayesian statistics due to their inherent limitations.
More recent data suggest a similar clinical performance between biodegradable polymer drug-eluting stents (BioMatrix™; Biosensors, Singapore; Nobori® and Ultimaster®; Terumo Corp, Tokyo, Japan; Orsiro; Biotronik, Bülach, Switzerland) and best-in-class durable polymer EES in “all-comers” trials at one-year follow-up (Figure 1). In addition, studies have shown that intervention with biodegradable polymer DES improved clinical outcomes, especially in patients with ST-segment elevation myocardial infarction5. Further direct comparisons of biodegradable polymer DES versus biocompatible polymer DES in randomised trials are awaited to evaluate appropriately the potential long-term benefits of biodegradable polymer DES after their polymer degradation.
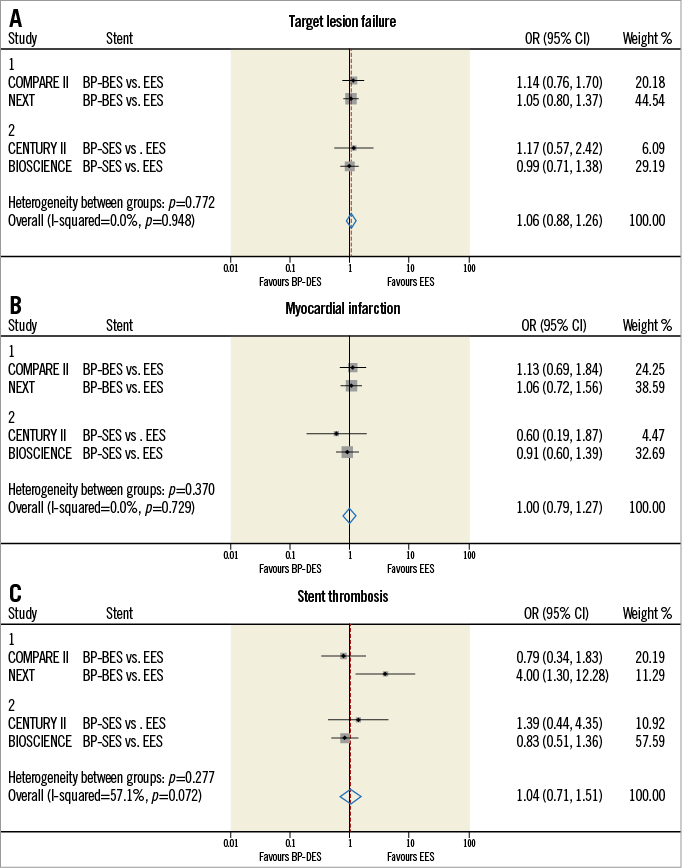
Figure 1. Meta-analyses of clinical outcomes between BP-DES and DP-EES in “all-comers” randomised trials. The pooled results show that no significant differences were found in relation to target lesion failure (A), myocardial infarction (B), and definite or probable stent thrombosis (C) between BP BES/SES and “best-in-class” DP-EES. BES: biolimus-eluting stent; BP: biodegradable polymer; DES: drug-eluting stent; DP: durable polymer; EES: everolimus-eluting stent; SES: sirolimus-eluting stent
In this issue of EuroIntervention, Vlachojannis et al report the long-term clinical outcomes comparing a biodegradable polymer-coated biolimus-eluting stent (BES) (Nobori; Terumo Corp) to the “gold standard” durable polymer-coated EES (XIENCE V or PRIME; Abbott Vascular, Santa Clara, CA, USA) among 2,707 patients in the COMPARE II trial6. The authors found similar safety and efficacy between biodegradable polymer BES and durable polymer EES in an all-comers population at three-year follow-up. Moreover, the one-year landmark analysis showed no significant differences in incremental event rates between one and three-year follow-up. One could expect that biodegradable polymer DES may have a potentially low risk of stent thrombosis after one-year follow-up; however, there were no safety or efficacy benefits seen with BES, over and above those with EES, beyond the first year. The reported rates of stent thrombosis between one and three years in both groups are pretty low (BES 0.47% vs. EES 0.34%, p=0.65). Although these findings highlight that both biodegradable polymer BES and biocompatible polymer EES had a very low risk of very late stent thrombosis, one may argue that the trial was underpowered to compare such a low incidence of adverse events. Therefore, either clinical trials with a larger sample size or imaging studies evaluating a surrogate of stent thrombosis are warranted.
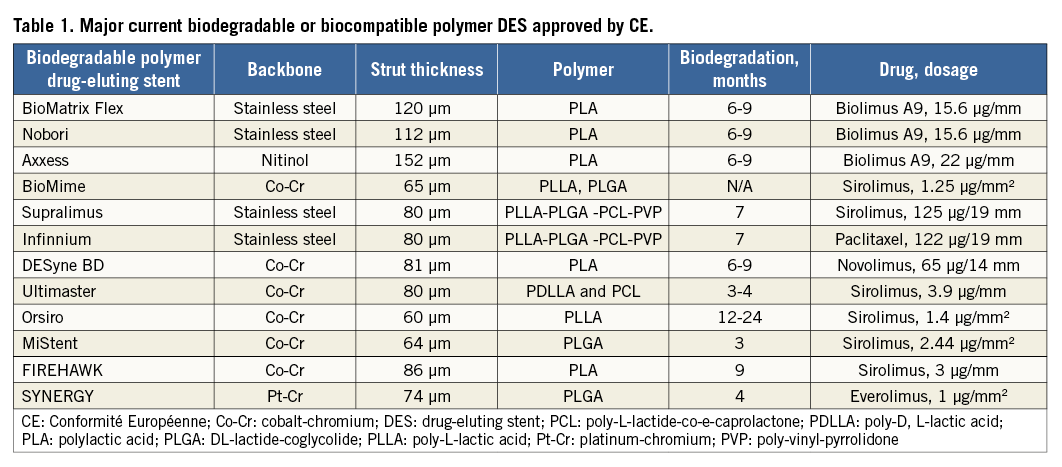
In the MOST trial, Parodi et al have reported that subacute stent thrombosis was mainly attributed to a significant stent underexpansion while late/very late stent thrombosis was mostly due to high proportions of uncovered and malapposed struts7. First, in terms of device-related stent thrombosis, it seems that post-dilation or guidance by intravascular ultrasound to reduce malapposition during the procedure is helpful to decrease subacute stent thrombosis. According to these important findings, low percentages of uncovered and malapposed struts at follow-ups partially represent a low risk of stent thrombosis after DES implantation. In the OCT substudy of the NEXT trial, incomplete vascular healing characterised by the presence of uncovered and malapposed struts was less common in EES compared with BES8. The authors explained that the favourable vascular healing in EES might be attributed to several factors such as biocompatible polymer with less inflammatory reactions, thin stent struts with more rapid healing, and flexible stent design with more adequate apposition. Therefore, a stent design with an optimal combination of materials, platform, polymer and drug is essential to achieve a better clinical performance (Table 1).
Recently, several biodegradable polymer DES with a novel design have shown good safety and efficacy in the treatment of patients with coronary artery disease. The BIOSCIENCE trial, comparing a biodegradable polymer sirolimus-eluting stent (SES) Orsiro against EES, demonstrated superiority of the biodegradable polymer SES to EES in terms of a significant reduction in the primary endpoint of target lesion failure defined as the composite of cardiac death, target vessel myocardial infarction, and clinically indicated target lesion revascularisation at 12-month follow-up (3.3% vs. 8.7%, p=0.024)5. In addition, the TARGET serial trials also showed encouraging clinical performance of a novel groove-filled biodegradable polymer SES, Firehawk® (MicroPort, Shanghai, China)9. Furthermore, with better early vascular healing, the duration of dual antiplatelet therapy can potentially be shortened after newer-generation DES implantation, whether with biodegradable or biocompatible polymer. This indeed has been endorsed in the recent revascularisation guidelines10.
Conflict of interest statement
The authors have no conflicts of interest to declare.

