Drug-eluting stents (DES) minimise the risk of in-stent restenosis by means of controlled, local delivery of antiproliferative drugs from a thin layer of coating applied to a rigid stent backbone. In this respect, polymer coatings have proven largely indispensable for the control of drug release kinetics and the optimisation of DES efficacy. Investigation of polymer-free stent platforms has typically yielded comparatively lower levels of neointimal suppression by virtue of rapid drug dissociation in the initial hours and days after stent implantation1. Indeed, all of the currently available FDA-approved DES devices control drug release by use of durable polymer coatings2,3. On the other hand, polymer coatings may elicit unanticipated off-target effects –ranging from eosinophilic hypersensitivity to foreign body reactions at one end of the spectrum4, to potentially favourable effects on reducing stent thrombogenicity in the acute phase5.
Until recently, the publicly accessible literature provided only limited benchtop data on the physical characteristics and surface integrity of different polymer coatings on devices in everyday use. Such data might be clinically important for a number of reasons. First, DES thrombogenicity could potentially be increased at regions of stent surface irregularity, due to inhomogeneous distribution or displacement of polymer coating. Coarse DES coating irregularities might promote the inflammatory reactions sometimes seen after DES implantation (Figure 1A and Figure 1B), which in turn act as a direct nidus for platelet activation and stent thrombosis. Second, the antiproliferative potential of DES might be locally reduced at sites of major coating loss: at such regions the DES is effectively a bare metal stent. Third, downstream microembolism of detached fragments of DES coating could lead to myocardial injury or infarction. In the current issue of EuroIntervention, a study from United States researchers addresses the issue of DES coating irregularities and free particle formation after stent expansion6.
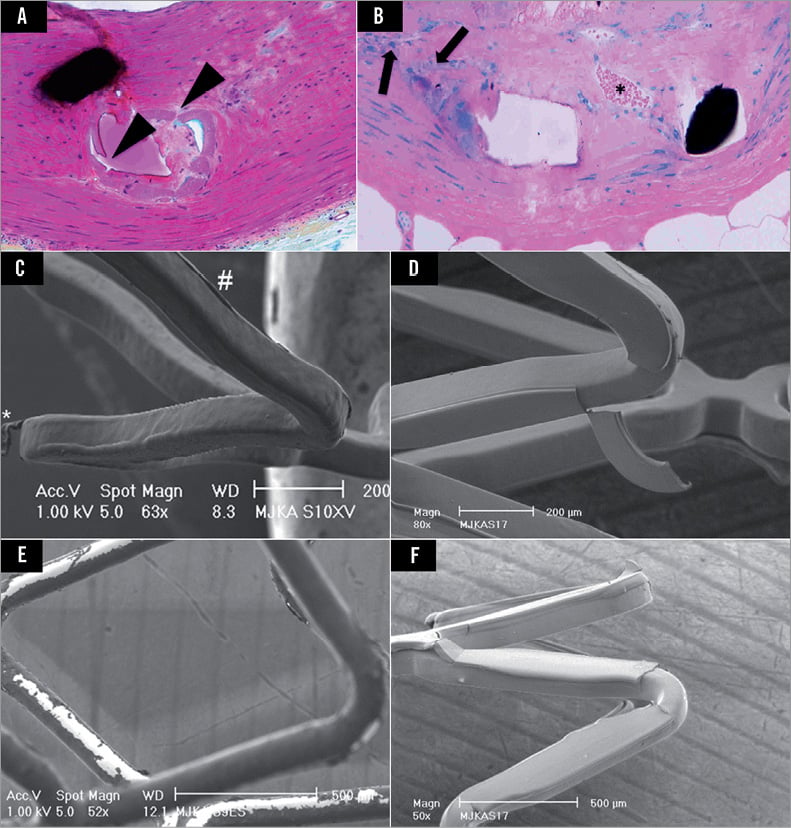
Figure 1. Representative high power (×200) magnifications of polymer-coated stent struts after Movat Pentachrome (A) and Giemsa eosin (B) staining. Note, there is presence of polymer cracking (black arrowheads) and delamination (black arrows) resulting in moderate inflammatory reaction, mostly consisting of monocyte and neutrophil infiltration. Furthermore, neovascularisation (*) is observed in the surroundings of stent struts as a sign of sustained inflammation. C) Partially detached fragment of coating (*) and ridge-like thickening of coating (#) on an everolimus-eluting XIENCE V® stent (Abbott Vascular, Santa Clara, CA, USA). D) Partially detached fragment of coating on a biolimus-eluting BioMatrix™ stent (size 85×310 µm; Biosensors International, Singapore, Singapore). E) Phosphorylcholine-based zotarolimus-eluting Endeavor® stent (Medtronic, Minneapolis, MN, USA) with visual aspect of bare metal areas. F) An area with total loss of coating on a biolimus-eluting stent, indicating total detachment of a polymer fragment. (Panels C-F modified from references 10 and 11).
The main findings extend observations from an earlier brief report7 and are scientifically interesting: the expansion of stent delivery balloons topographically disturbs the polymer surfaces of all examined DES devices, and this disturbance can be complicated by the liberation of microparticles which the investigators collected from a filtered expansion chamber.
DES coating irregularities and fragments reported in the literature
Thus far, a limited body of research data has examined microscopic morphology, coating irregularities or physical properties of polymer-based DES devices8-11. An initial systematic classification and quantification of coating irregularities on the surface of various types of DES reported in 2009 showed that the incidence and size of various coating irregularities differed widely among different types of DES11. The present carefully executed study of Denardo and co-workers builds further on the available literature and confirms that important qualitative and quantitative differences in surface coating exist among approved DES platforms that are in routine clinical use6. Earlier research on expanded (and post-dilated) DES demonstrated loosely attached polymer particles with a very wide range of size, e.g., approximately 30 µm on a durable polymer-based DES coating (Figure 1C) versus up to 300 µm on a biodegradable polymer-based DES coating (Figure 1D)10,11. (These differences should not be considered as polymer class effects; preliminary data suggest that there may be equally important differences in how different biodegradable polymer coatings react upon stent expansion)12. In addition, the identification of uncoated areas on DES may be considered as evidence of total detachment of polymer fragments (Figure 1E and Figure 1F), in particular if interpreted in the context of previously reported data from unexpanded DES13. In the present study, Denardo and co-workers went one step further, collecting and analysing totally detached particles liberated during DES expansion, by filtering the medium in which the DES were expanded. Optical microscopy and scanning electron microscopy (SEM) were used for subsequent examination of the filter, aiming at qualitative and quantitative analysis of the free particles captured. In interpreting their findings, the technical challenges of the work and the translational relevance of the data should be considered.
Technical challenges of the benchtop assessment of DES surfaces
The report of Denardo et al primarily quantified stent coating irregularities based on optical microscopy, which allowed rapid handling of the samples. However, in contrast, most of the recent studies by others used systematic electron microscopy examination due to its three-dimensional properties and its capacity to obtain high magnification (>100,000-fold) images with high spatial resolution8-11. In addition, the FDA specifically mention SEM-based examination for visualisation of acute polymer injury in published draft guidance for industry14. Certainly, minor topographical irregularities related to manufacturing may be missed by optical microscopy, such as waviness, flattening and cratering of the polymer, as well as coating adhesion (a factor in subsequent polymer webbing observed for example with the TAXUS™ stent; Boston Scientific, Natick, MA, USA). On the other hand, major polymer damage such as coating delamination, peeling and ridging is likely to be identified with both methodologies.
A number of additional technical factors deserve consideration. First, the impact of interobserver variability must be acknowledged. Adjudication of irregularities is reliant on an analyst’s experience and judgement and thus entails a certain degree of subjectivity. Semi-automated tools with image analysis software could increase reproducibility and facilitate meaningful comparisons between the findings of different research groups. Second, the FDA currently suggests in a non-binding recommendation the use of a robust number of stents from multiple stent lots for each test (i.e., a minimum of three batches)14, but the optimal number of stent samples required for individual experiments is not known. In addition, it is desirable that a minimum stent surface area is examined in order not to miss certain irregularities; this may be even more important with newer-generation stents that appear to show fewer coating irregularities. Third, stent expansion in an aqueous medium followed by drying could theoretically create artificial cracking and splitting, preferentially affecting more hydrophilic coatings. This effect can be minimised by gradual passive drying without temperature changes, which seems to be the method employed by the authors. The use of environmental SEM might theoretically avoid the problem to some extent, but this imaging technique is very time-consuming, and less suitable for studies utilising stepwise scanning of relatively large surface area cylindrically shaped stent samples that have to be turned repeatedly.
Translational relevance of these findings?
Of course, benchtop studies do not accurately mimic the complex interplay of individual stent components, delivery devices and disease conditions in vivo. Indeed, an important additional feature of the bench model of Denardo et al is that the stents were expanded in a fluid medium without utilisation of a vessel phantom. Benchtop testing of DES surfaces is mostly performed without the use of phantoms, as careful examination of the stent surface is impossible inside the phantom, and extraction of the sample from the phantom may increase the frequency and size of coating irregularities. One consequence of this approach is that the known impact of abluminal coating damage due to contact with the vessel wall as well as tracking to the lesion is not accounted for9. On the other hand, some investigators have shown reduction in balloon expansion-related polymer damage when stents are expanded in phantoms with compliance similar to that of human coronary arteries15. Moreover, in clinical use the majority of abluminally derived particles liberated upon balloon expansion may remain trapped in the vessel wall behind the implanted stent. Thus the local vessel wall impact of microparticles (e.g., local inflammation, endothelial dysfunction) might be more clinically important than downstream effects (such as microvascular obstruction).
In this respect, historical preclinical studies have highlighted the problem of delayed arterial healing after DES implantation and the fact that the prolonged inflammation observed after first and second-generation devices is most likely secondary to polymer residues, especially in overlapping stented sections16. Infiltration of neutrophils and eosinophils was clearly increased in first-generation DES compared to uncoated metal stents, and the pro-inflammatory contribution of damaged polymers and delamination products in this process is probably significant (Figure 1A and Figure 1B). In fact, the clinical implication of damaged durable polymers may have been under-recognised and may have a substantial impact on clinical outcomes of patients receiving these stents.
Clinical evidence supporting the importance of topographical irregularities is difficult to evaluate. There are two points of view. On the one hand, it can be argued that the differences in polymer irregularities and microparticle formation among stents might have contributed to differences in periprocedural myocardial infarction observed in some randomised clinical trials (e.g., paclitaxel-eluting versus everolimus-eluting stents17, and biolimus A9-eluting versus sirolimus-eluting stents18) as well as to the lower rates of stent thrombosis observed with newer-generation durable polymer DES19. Nevertheless, linking such findings to free particle formation during stent deployment only may be too simplistic, as various other DES or patient-related factors may play a role20. Indeed, it might equally be observed that the somewhat “less favourable” appearance of some DES on the bench did not translate into detectable safety issues in large-scale randomised trials10,21. It may well be that the advantages of stent macro-components (such as drug load and release kinetics, and stent superstructure) outweigh the impact of micro-components (such as surface irregularity and microparticle liberation). Accordingly, while we should be grateful for the important contribution of researchers such as Denardo and colleagues, more data are needed before we can be sure of the clinical relevance of the polymer irregularities and microparticles identified in bench studies.
Perspective
The concept of covering DES with polymer-based coatings turned out to embody both “Samson’s hair” and “Achilles’ heel” of these devices. While polymer coatings have a central role in ensuring the antirestenotic efficacy of DES devices –and may even have an acute protective role in reducing thrombogenicity– these advantages occur at the collateral cost of a significant delay in arterial healing in comparison with uncoated stents, and an associated spectrum of clinicopathological events including late thrombotic stent occlusion. The present report of Denardo et al6 sheds further light on the issue of balloon expansion-induced polymer disruption and microparticle liberation, an effect that appeared to vary among DES types studied. Although the clinical relevance of these findings remains to be fully elucidated, the potential relevance is such that in our opinion detailed bench evaluation of polymer coating integrity should be incorporated into European regulatory body approval processes, as is the case in the United States. In addition, we propose that the use of standardised methodology and reporting, including systematic SEM examination of coating irregularities, will allow meaningful comparison of findings between studies. Further research on DES devices that are in widespread clinical use will be of great interest to the interventional cardiology community. The publication of such data in the peer-reviewed literature would represent an important contribution to the further development of this technology.
Funding
The research department of Thoraxcentrum Twente has received educational and/or research grants funded by Abbott Vascular, Biotronik, Boston Scientific, and Medtronic.
Conflict of interest statement
C. von Birgelen is a consultant to and has received lecture fees or travel expenses from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. The other authors have no conflicts of interest to declare.

