Abstract
Background: Recent clinical studies are testing strategies for short (1-3 months) dual antiplatelet therapy following newer-generation drug-eluting stent (DES) placement. However, detailed biological responses to newer-generation DES remain unknown in humans.
Aims: We sought to evaluate early pathologic responses to abluminal biodegradable polymer-coated (BP-) DES compared with circumferential durable polymer-coated (DP-) DES in human autopsy cases.
Methods: The study included 38 coronary lesions with newer-generation DES implanted for <90 days (DP-DES=24, BP-DES=14) in 26 autopsy cases. The degree of strut coverage was defined as follows: grade 0 (bare), grade 1 (with fibrin or tissues/cells without endothelium), grade 2 (with single-layered endothelium), and grade 3 (with endothelium and underlying smooth muscle cell layers).
Results: The duration following implantation was similar in DP- and BP-DES (median=20 vs 17 days). A total of 2,022 struts (DP-DES=1,297, BP-DES=725) were pathologically analysed. Focal grade 2 coverage was observed as early as 5 days after the implantation in both stents. The multilevel mixed-effects ordered logistic regression model demonstrated that BP-DES exhibited greater strut coverage compared with DP-DES (odds ratio [OR]: 3.64, 95% confidence interval [CI]: 1.37-9.67; p=0.009), which remained significant after adjustment for the duration following implantation and underlying tissue characteristics (OR: 2.74, 95% CI: 1.10-6.80; p=0.030). The predictive probability of grade 2 and 3 coverage was comparably limited at 30 days (DP-DES=17.1%, BP-DES=28.7%) and increased at 90 days (DP-DES=76.5%, BP-DES=86.6%). Both stents showed low inflammation and a similar degree of fibrin deposition.
Conclusions: Single-layered endothelial coverage begins in the days after newer-generation DES placement, and BP-DES potentially exhibit faster strut coverage with smooth muscle cell infiltration than DP-DES in humans. Nevertheless, vessel healing remains suboptimal in both stents at 30 days.
Introduction
Dual antiplatelet therapy (DAPT) is a cornerstone of antithrombotic treatment in patients undergoing percutaneous coronary intervention. The optimal duration of DAPT (i.e., the minimal period needed to ensure the best safety and efficacy) for preventing ischaemic complications, including stent thrombosis, has been extensively explored in randomised controlled trials over the last few years123. The accumulating evidence supports a clinical approach in which the bleeding risk plays a prevailing role: in patients at high bleeding risk, it is generally advisable to reduce the duration of DAPT irrespective of their risk of thrombosis23. Therefore, recent clinical studies are testing strategies for short (1-3 months) DAPT regimens123.
We have previously reported pathological responses to circumferential durable polymer-coated (DP-) cobalt-chromium everolimus-eluting stents in human autopsy cases, in which strut coverage remained suboptimal for a duration of 1 to 3 months after implantation, as 60% of lesions had substantially uncovered struts4. The thin-strut abluminal biodegradable polymer-coated (BP-) drug-eluting stent (DES) was developed to promote early re-endothelialisation and achieve fast vessel healing, both of which potentially contribute to long-term vessel adaptation as well as reduced early stent failure5. The safety and effectiveness of BP-DES have been shown in many clinical trials35. Nevertheless, biological responses to BP-DES, including the process of vessel healing and the degree of inflammation and fibrin deposition, remain uncertain because there has been limited pathological assessment of BP-DES in humans. Importantly, vascular responses to BP-DES may be different from those to DP-DES, particularly in the early phase following the implantation due to the difference in polymer coating style and stent design.
We therefore investigated pathologic responses to newer-generation DES in the early phase (<90 days) following implantation by comparing BP-DES with DP-DES in human coronary arteries using a registry of autopsy cases. In the present study, the process of stent healing was precisely evaluated for every single strut in association with underlying tissue characteristics and analysed with detailed and accurate clinical information.
Methods
Patient and lesion selection
Between January 2007 and April 2020, the department of pathology at the National Cerebral and Cardiovascular Center (Suita, Japan) received 741 autopsy cases of patients who died at the institution, including 82 cases with a total of 143 stented coronary lesions. In addition, the department received 36 autopsy cases with a total of 63 stented coronary lesions from other hospitals in Japan for pathological assessment. Consequently, the National Cerebral and Cardiovascular Center stent autopsy registry had a total of 206 stented coronary lesions in 118 autopsy cases. Of these, all available coronary lesions with thin-strut newer-generation DES (either circumferential DP-DES or abluminal BP-DES) implanted for <90 days and obtained from adult autopsy cases (≥18 years old) were included in the study. Thick-strut BP-DES (Nobori; Terumo), a limited number of circumferential BP-DES (Orsiro; BIOTRONIK), and newer-generation DES implanted for ≥90 days were excluded from the study in order to focus on early vascular responses to thin-strut abluminal BP-DES compared with circumferential DP-DES with a similar duration following implantation (Figure 1). All specimens had already been prepared during previous pathological assessment.
Overlapped or consecutively implanted stents were treated as 1 lesion, while stents with a gap of >5 mm were considered to be separate lesions6. Clinical records were reviewed for patient history, risk factors, medications, procedural information including stent diameter and length, and accurate duration of implantation. Cause of death was reported as stent-related cardiac death, non-stent-related cardiac death, or non-cardiac death, as previously described6. The study protocol was approved by the institutional ethics committee of the National Cerebral and Cardiovascular Center (research project number: R20098-2).
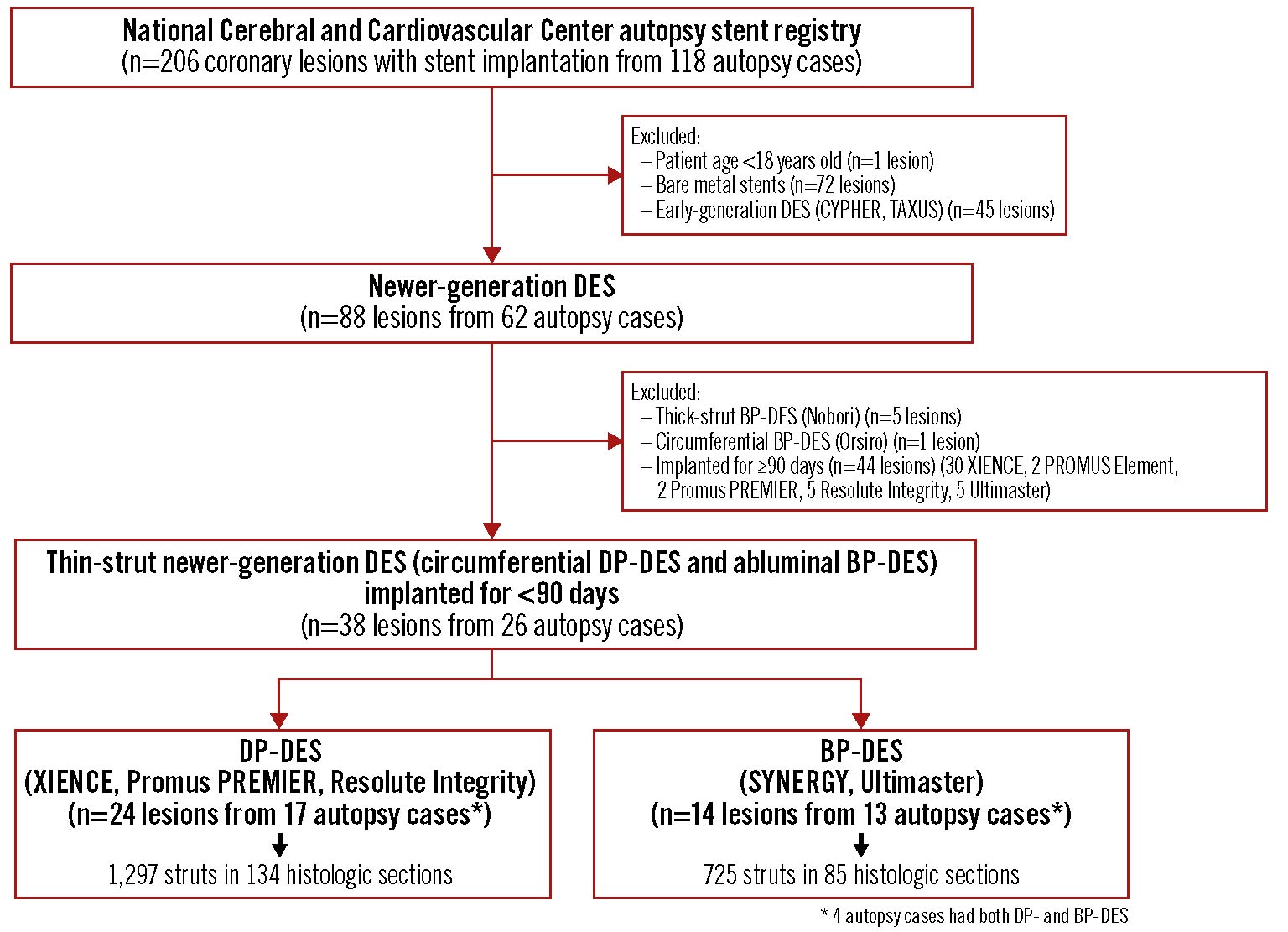
Figure 1. Patient and lesion selection scheme. CYPHER (Cordis), TAXUS (Boston Scientific), PROMUS Element (Boston Scientific). BP-DES: biodegradable polymer-coated drug-eluting stent; DES: drug-eluting stent; DP-DES: durable polymer-coated drug-eluting stent
Histological preparation
Hearts were fixed in 10% neutral buffered formalin. Epicardial coronary arteries were dissected from the heart, and the stented segments were embedded in hydroxyethyl methacrylate. The entire stent was sawed serially at 5 mm intervals. Histological sections were cut at 5 μm and stained with haematoxylin and eosin, Masson’s trichrome, and Elastica van Gieson.
Pathological assessment and morphometric analysis
The underlying plaque morphology (outside stent struts) for each lesion was classified using traditional definitions of pathological intimal thickening, fibroatheroma, thin-cap fibroatheroma, plaque rupture, calcified nodule, and fibrocalcific plaque7. Morphological features of calcification in the stented lesion were classified into fragmented, sheet-like, or nodular calcification7. The severity of calcification was evaluated based on the extent of calcification in histological sections and classified as none, mild (focal and detected in one section), moderate (detected in two or more sections but <75% of the lesion), and severe (detected in ≥75% of the lesion).
Morphometric measurements were performed using image analysis software (cellSens; Olympus) for the external elastic lamina, internal external lamina and lumen areas. The degree of stent strut coverage was classified into 4 categories and defined as follows: grade 0, bare struts; grade 1, struts covered with thrombus, fibrin, or other tissues or cells without endothelium; grade 2, struts covered with single-layered endothelium without underlying smooth muscle cell (SMC) layers; and grade 3, struts covered with endothelium and underlying SMC layers (Figure 2). The degree of strut coverage was evaluated for every single strut, where underlying tissue characteristics (just behind the stent strut) were also evaluated for each strut and classified as fibrous tissue, necrotic core, lipid pool, calcification, thrombus, another stent strut, and incomplete stent apposition. The degree of fibrin deposition was evaluated as the percentage of struts with fibrin, and the extent of inflammation was assessed using a grading scale of 0 to 4 as previously described4. The percentage of stent struts with giant cells and eosinophils, and the frequencies of tissue protrusion and medial disruption were also evaluated4. Immunohistochemistry was carried out in select cases using an anti-alpha smooth muscle actin (ɑ-SMA) antibody (dilution 1:10; Dako) for SMC. The slides were incubated with primary antibodies, followed by a mouse-rabbit-horseradish peroxidase polymer and visualised with a 3,3’-diaminobenzidine substrate with haematoxylin as a counterstain (Leica Microsystems).
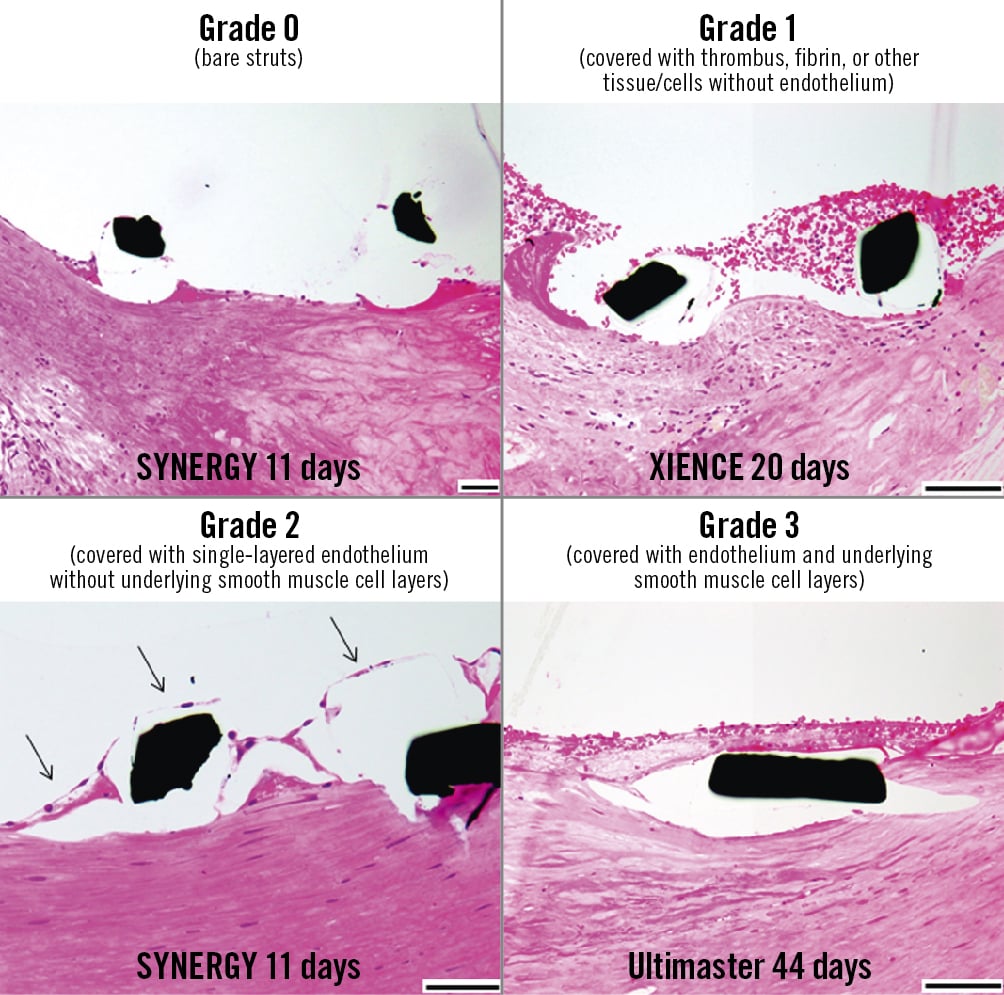
Figure 2. Classification of strut coverage. Arrows indicate endothelium. Haematoxylin and eosin staining. Black scale bars=100 μm.
Statistical analysis
Results for continuous variables with normal distribution were expressed as mean±standard deviation (SD). Variables with non-normal distribution were expressed as median and 25th to 75th percentiles. For per-patient and per-lesion analyses, comparisons of continuous variables with normal distribution were analysed with the Student’s t-test, those with non-normal distribution were tested by the Wilcoxon rank-sum test, and categorical variables were analysed by the chi-square test. For per-strut analysis, we used a multilevel mixed-effects ordered logistic regression model to investigate the difference in the degree of stent strut coverage (an ordered dependent variable) between the DP- and BP-DES groups. We have considered patient and lesion levels as random effects in the model. In addition, the comparison of the degree of strut coverage between the groups in the per-strut analysis was adjusted for the duration following implantation and underlying tissue characteristics (fibrous tissues, necrotic core, calcification, and incomplete stent apposition), both of which have been shown to substantially impact the process of stent healing4. Finally, the predictive probability of strut coverage stratified by the duration following implantation in DP- and BP-DES was analysed by multilevel mixed-effects ordered logistic regression. All analyses were performed with JMP 14 (JMP) and Stata 16.1 (StataCorp). The statistical tests were 2-tailed, and a p-value <0.05 was considered to indicate statistical significance.
Results
Study population
Of the 206 stented coronary lesions in 118 autopsy cases, 88 lesions in 62 autopsy cases had newer-generation DES. After excluding lesions with thick-strut BP-DES (Nobori; n=5), a limited number of circumferential BP-DES (Orsiro; n=1), and DES implanted for ≥90 days (n=44; 39 DP-DES and 5 BP-DES), a total of 38 coronary lesions with thin-strut newer-generation DES implanted for <90 days in 26 autopsy cases were finally included in the study. The investigated lesions with newer-generation DES consisted of 24 with DP-DES (18 XIENCE [Abbott Vascular], 1 Promus PREMIER [Boston Scientific], 5 Resolute Integrity [Medtronic]) and 14 with BP-DES (9 SYNERGY [Boston Scientific], 5 Ultimaster [Terumo]) (Figure 1). The principal characteristics of the investigated DES are described in Supplementary Figure 1.
Patient and lesion characteristics
Patient characteristics including age, gender, and risk factors were comparable between the DP- and BP-DES groups. In the majority of cases in both groups, clinical presentation was acute coronary syndrome (ACS). All subjects but one in the BP-DES group were taking aspirin, and the majority of patients were on P2Y12 receptor inhibitors (Table 1). Cause of death was mostly non-stent-related cardiac death (especially due to heart failure), and there were two cases (1 in each group) of non-cardiac death (pneumonia and ischaemic colitis, respectively) (Table 1). There was one case showing focal thrombus formation within the stent in the BP-DES group (Ultimaster implanted for acute myocardial infarction for 5 days); however, the cause of death of the patient was cardiac rupture associated with the initial acute myocardial infarction. No patient died of stent-related causes (in-stent restenosis or stent thrombosis).
The duration following implantation was similar between DP- and BP-DES (median [25th to 75th percentiles]: 20 [12-27] vs 17 [5-46] days; p=0.88) (Table 1, Supplementary Figure 2). The predominant indication for stenting was ACS (culprit lesions) in both groups (58% in DP-DES and 79% in BP-DES), and only a few lesions were stented for chronic coronary syndromes (CCS). There were no differences in lesion location, number of stents per lesion, stent length and diameter, and frequency of overlapping stents between the groups. Since the SYNERGY stent has different strut thicknesses for different diameters (Supplementary Figure 1), the mean strut thickness including polymer coating was recalculated as per length, and was significantly thicker for DP-DES than BP-DES (97.8±1.6 vs 86.1±7.2 μm/mm; p<0.0001). Underlying plaque morphology was mostly plaque rupture or fibroatheroma in both groups. More than half of the lesions in both groups contained moderate or severe calcification, where sheet-like calcification was the most frequent morphology of calcification (Table 1). Patient and lesion characteristics for the 4 different types of the investigated DES are shown in Supplementary Table 1, in which XIENCE and Promus PREMIER were considered to be similar types of DES.
Table 1. Patient and lesion characteristics and histopathologic analysis.
| Patient characteristics | DP-DES | BP-DES | p-value | |
|---|---|---|---|---|
| n=17 autopsy cases* | n=13 autopsy cases* | |||
| Age (years) | 76±15 | 72±15 | 0.45 | |
| Male gender | 10 (59%) | 9 (69%) | 0.56 | |
| Hypertension | 9 (53%) | 9 (69%) | 0.37 | |
| Dyslipidaemia | 11 (65%) | 10 (77%) | 0.47 | |
| Diabetes mellitus | 6 (35%) | 6 (46%) | 0.55 | |
| Clinical presentation | ACS | 16 (94%) | 12 (92%) | 0.84 |
| CCS | 1 (6%) | 1 (8%) | ||
| Aspirin | 17 (100%) | 12 (92%) | 0.24 | |
| P2Y12 receptor inhibitors | 15 (88%) | 11 (85%) | 0.77 | |
| Cause of death | Stent-related | 0 (0%) | 0 (0%) | 0.84 |
| Non-stent-related cardiac | 16 (94%) | 12 (92%) | ||
| Heart failure | 11 (65%) | 9 (69%) | ||
| Ventricular arrhythmia | 2 (12%) | 2 (15%) | ||
| Cardiac rupture | 3 (18%) | 1 (8%) | ||
| Non-cardiac | 1 (6%) | 1 (8%) | ||
| Pneumonia | 1 (6%) | 0 (0%) | ||
| Ischaemic colitis | 0 (0%) | 1 (8%) | ||
| Lesion characteristics | n=24 lesions | n=14 lesions | p-value | |
| Type of DES | XIENCE | 18 (75%) | 0 | – |
| Promus PREMIER | 1 (4%) | 0 | ||
| Resolute Integrity | 5 (21%) | 0 | ||
| SYNERGY | 0 | 9 (64%) | ||
| Ultimaster | 0 | 5 (36%) | ||
| Duration following implantation (days) | 20 (12-27) | 17 (5-46) | 0.88 | |
| Indication for stenting | ACS (culprit lesion) | 14 (58%) | 11 (79%) | 0.41 |
| ACS (non-culprit lesion) | 5 (21%) | 2 (14%) | ||
| CCS | 5 (21%) | 1 (7%) | ||
| Lesion location | LM/LAD/LCx/RCA/graft | 4/8†/2/10/0 | 2/6†/2/3/1 | 0.51 |
| Number of stents per lesion | 1.3±0.6 | 1.4±0.5 | 0.61 | |
| Stent length (mm) | 30.9±23.8 | 34.0±16.8 | 0.67 | |
| Stent diameter (mm) | 3.1±0.5 | 3.0±0.5 | 0.61 | |
| Overlapping stents | 7 (29%) | 6 (43%) | 0.39 | |
| Underlying plaque morphology | Rupture/TCFA/FA/PIT/FC/CN/dissection | 10/1/9/1/2/1/ 0 | 6/0/3/1/2/1/1 | 0.73 |
| Lesion calcification | None/mild/moderate/severe | 1/10/3/10 | 0/4/1/9 | 0.54 |
| Morphology of calcification | Fragmented/sheet-like/nodular | 9/13/1 | 4/7/3 | 0.34 |
| Histopathological analysis (lesion level) | n=24 lesions | n=14 lesions | p-value | |
| Number of histological sections evaluated | 134 | 85 | – | |
| Number of struts evaluated | 1,297 | 725 | – | |
| External elastic lamina area (mm2) | 17.6±7.4 | 17.6±6.9 | 0.99 | |
| Internal elastic lamina area (mm2) | 15.1±6.5 | 14.6±5.7 | 0.80 | |
| Lumen area (mm2) | 6.5±2.1 | 7.5±3.0 | 0.23 | |
| Struts with fibrin (%) | 88.3 (66.4-100.0) | 96.4 (83.3-100.0) | 0.37 | |
| Inflammation score | 0.07 (0-0.33) | 0 (0-0.47) | 0.95 | |
| Struts with eosinophils (%) | 0 (0-0) | 0 (0-0.77) | 0.40 | |
| Struts with giant cells (%) | 4.0 (0-11.7) | 3.2 (0-11.0) | 0.58 | |
| Prevalence of tissue protrusion | 11 (46%) | 5 (36%) | 0.54 | |
| Prevalence of medial disruption | 14 (58%) | 8 (57%) | 0.94 | |
| Histopathological analysis (strut level) | n=1,297 struts | n=725 struts | p-value | |
| Strut coverage | Grade 0 | 559 (43%) | 254 (35%) | 0.009‡ |
| Grade 1 | 612 (47%) | 291 (40%) | ||
| Grade 2 | 102 (8%) | 152 (21%) | ||
| Grade 3 | 24 (2%) | 28 (4%) | ||
| Underlying tissue characteristics | Fibrous tissue | 1,079 (83%) | 612 (84%) | – |
| Necrotic core | 80 (6%) | 32 (4%) | – | |
| Lipid pool | 0 (0%) | 3 (0.4%) | – | |
| Calcification | 89 (7%) | 43 (6%) | – | |
| Thrombus | 2 (0.2%) | 0 (0%) | – | |
| Stent strut | 0 (0%) | 4 (0.6%) | – | |
| Incomplete stent apposition | 47 (4%) | 31 (4%) | – | |
| Values are expressed as mean±SD, median (25th to 75th percentiles), or n (%). *4 patients had both DP- and BP-DES, and 1 patient had 2 different types of DP-DES (XIENCE and Resolute Integrity). †4 of 8 lesions in the DP-DES group and 2 of 6 lesions in the BP-DES group had crossover stenting from the left main to the LAD. ‡Statistical analysis was performed by multilevel mixed-effects ordered logistic regression analysis. ACS: acute coronary syndromes; BP-DES: biodegradable polymer-coated drug-eluting stents; CCS: chronic coronary syndromes; CN: calcified nodule; DES: drug-eluting stents; DP-DES: durable polymer-coated drug-eluting stents; FA: fibroatheroma; FC: fibrocalcific plaque; LAD: left anterior descending artery; LCx: left circumflex artery; LM: left main coronary artery; PIT: pathologic intimal thickening; RCA: right coronary artery; TCFA: thin-cap fibroatheroma | ||||
Morphometric analysis
Morphometric analysis was performed on a total of 219 histological sections (DP-DES=134, BP-DES=85) with 2,022 struts (DP-DES=1,297, BP-DES=725) in 38 lesions (DP-DES=24, BP-DES=14) (Table 1). There were no differences in the areas of the external elastic lamina, internal elastic lamina, and lumen between the groups. Approximately 40% of the lesions in both groups showed tissue protrusion, and medial disruption was observed in more than half of the lesions in both groups. The majority of the struts exhibited fibrin deposition, where the percentage of struts with fibrin was comparable between DP- and BP-DES. The degree of inflammation and eosinophil infiltration was low and similar in both groups. No difference in the percentage of struts with giant cells was observed between the groups (Table 1). No lesions exhibited in-stent neoatherosclerosis.
The degree of strut coverage in DP- and BP-DES
Representative histological images of DP- and BP-DES in the very early phase (within days to a month) following the implantation are shown in Figure 3. The majority (80 to 90%) of the struts in this phase were categorised as grade 0 (bare) or grade 1 (covered with thrombus or tissues without endothelium) for both DP- and BP-DES. However, focal grade 2 coverage (single-layered endothelial coverage) was observed as early as 3-5 days after the implantation in both DP- and BP-DES, with or without underlying thrombus or tissues (Figure 3). The earliest timepoint for grade 3 coverage (with endothelium and underlying SMC layers) was 5 days for BP-DES and 27 days for DP-DES. Although a substantial number of struts remained grade 0 or 1 for both DES implanted for >30 days, vessel healing progressed with time to increase the frequency of grade 2 and 3 coverage. Representative histological images of DP- and BP-DES at 1-3 months after the implantation are illustrated in Figure 4. Fibrin deposition remained observed in many struts showing grade 2 or 3 coverage, while SMC infiltration characterising grade 3 coverage was indeed confirmed by immunostaining for ɑ-SMA (Figure 4).
The overall observed frequencies of each grade of strut coverage (grade 0, 1, 2, and 3) in DP-DES were 43%, 47%, 8%, and 2%, and those in BP-DES were 35%, 40%, 21% and 4%, respectively (Table 1, Figure 5A). The multilevel mixed-effects ordered logistic regression model demonstrated that BP-DES exhibited greater strut coverage compared with DP-DES (odds ratio [OR]: 3.64, 95% confidence interval [CI]: 1.37-9.67; p=0.009) (Figure 5B). More than 80% of the struts in both groups had underlying fibrous tissues, and there was some underlying necrotic core, calcification, and incomplete stent apposition (Table 1). For struts with underlying necrotic core, the observed frequency of grade 2 coverage was greater in BP-DES than DP-DES, whereas for struts with underlying calcification and incomplete stent apposition, both DP- and BP-DES showed low frequencies of grade 2 and 3 coverage (Figure 4, Figure 5C). However, a limited number of struts with underlying necrotic core, calcification, and incomplete stent apposition did not allow the application of a multilevel mixed-effects ordered logistic regression model to compare the degree of strut coverage between DP- and BP-DES in each underlying tissue characteristic. Nevertheless, greater strut coverage in BP-DES compared with DP-DES was demonstrated by multilevel mixed-effects ordered logistic regression analysis even after adjustment for duration following implantation and underlying tissue characteristics (OR: 2.74, 95% CI: 1.10-6.80; p=0.030) (Figure 5B). When the degree of strut coverage in BP-DES was compared with fluoropolymer-coated DES (XIENCE and Promus PREMIER), the unadjusted odds ratio was 3.87 (95% CI: 1.37-10.91; p=0.011) and the adjusted OR was 2.72 (95% CI: 1.00-7.41; p=0.051). The results of pathological assessment and morphometric analysis for the 4 different types of the investigated DES are shown in Supplementary Table 1.
There were 4 cases that had both DP-DES (XIENCE) and BP-DES (SYNERGY). The duration following implantation was similar for DP- and BP-DES in each case. In 2 of those 4 cases with a duration of <14 days following implantation, the degree of strut coverage was substantially greater in BP-DES than DP-DES. For the remaining 2 cases, DP-DES showed a relatively greater frequency of grade 3 coverage than BP-DES, although the observed frequency of grade 2 and 3 coverage was similar for DP- and BP-DES (Supplementary Table 2).
The majority of the tissue protrusion consisted of the necrotic core in underlying plaque rupture (Figure 3, Supplementary Figure 3), and DP-DES showed a relatively low frequency of grade 0 and high frequency of grade 1 coverage in lesions with tissue protrusion. Nevertheless, the presence of tissue protrusion itself did not affect the observed frequency of grade 2 or 3 coverage in both DP- and BP-DES (Supplementary Figure 3). Although more than half of the lesions exhibited medial disruption, the presence of medial disruption had no apparent effect on early vessel healing in both stents (Supplementary Figure 3).
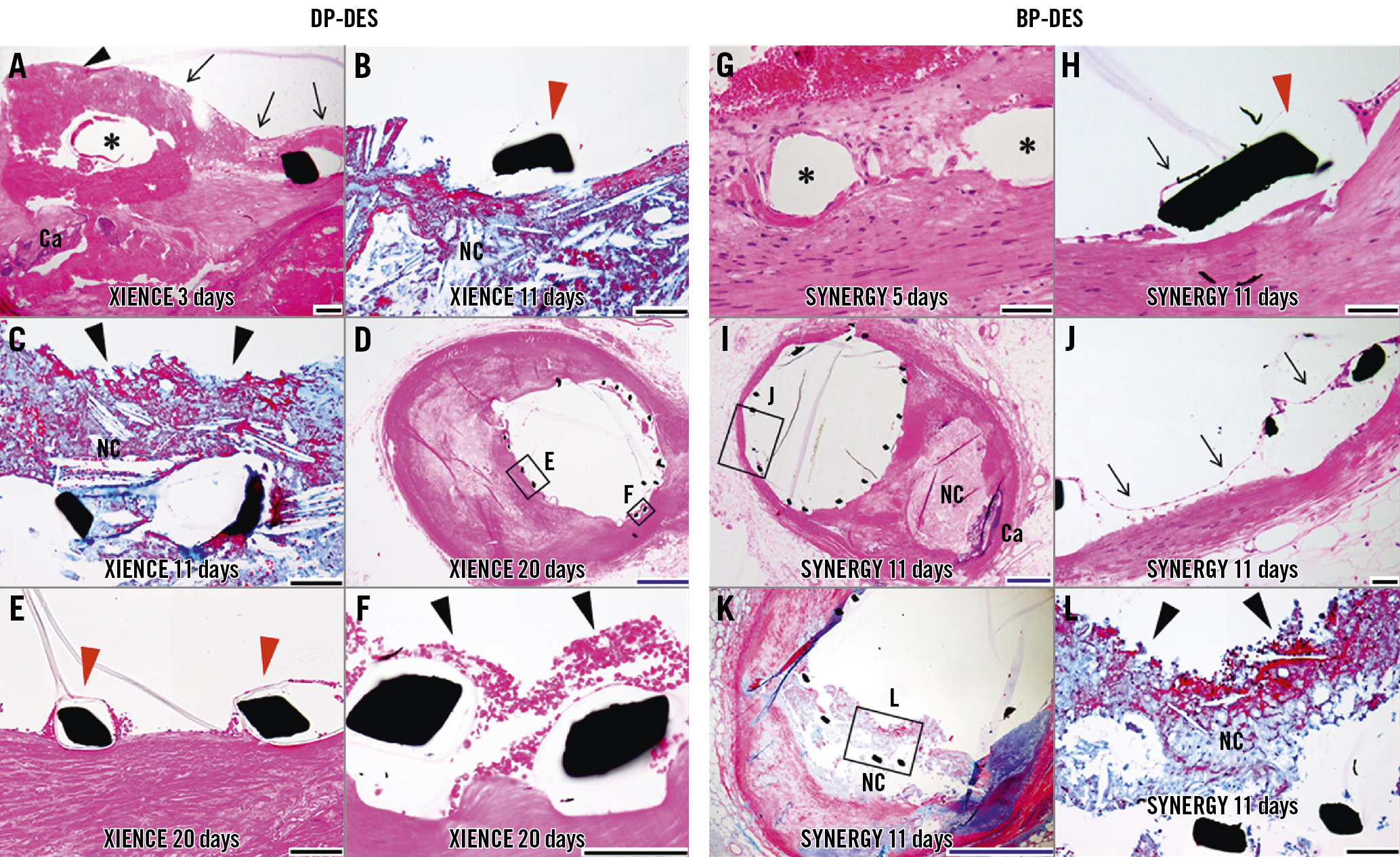
Figure 3. Representative histological images showing vascular responses to DP-DES (A-F) and BP-DES (G-L) at the very early phase (within days to a month) following the implantation. A) Peristrut fibrin thrombus without endothelium (black arrowhead=grade 1) on underlying calcification (Ca) and single-layered endothelial coverage (arrows=grade 2) with underlying fibrin thrombus. B-C) A bare strut (red arrowhead=grade 0) on the necrotic core (NC) and struts with overlying NC (tissue protrusion) without endothelium (black arrowhead). D-F) A low-power image (D) shows well-apposed struts, and high-power images depict bare struts (grade 0, E) and peristrut thrombus without endothelium (grade 1, F). G) Struts covered with endothelium and smooth muscle cell layers (grade 3). H-L) A high-power image in (H) shows a bare strut (red arrowhead) with partial endothelial coverage (arrow; grade 0). A low-power image in (I) shows a well-expanded stent with underlying NC, and a high-power image shows single-layered endothelial coverage (grade 2, J). A low-power image in (K) shows struts penetrated into the NC, and a high-power image shows struts with overlying NC (tissue protrusion) without endothelium (grade 1, L). Haematoxylin and eosin (A, D-J) and Masson’s trichrome staining (others). *Stent struts. Black scale bars=100 μm; Blue scale bars=1.0 mm. BP-DES: biodegradable polymer-coated drug-eluting stent; DP-DES: durable polymer-coated drug-eluting stent
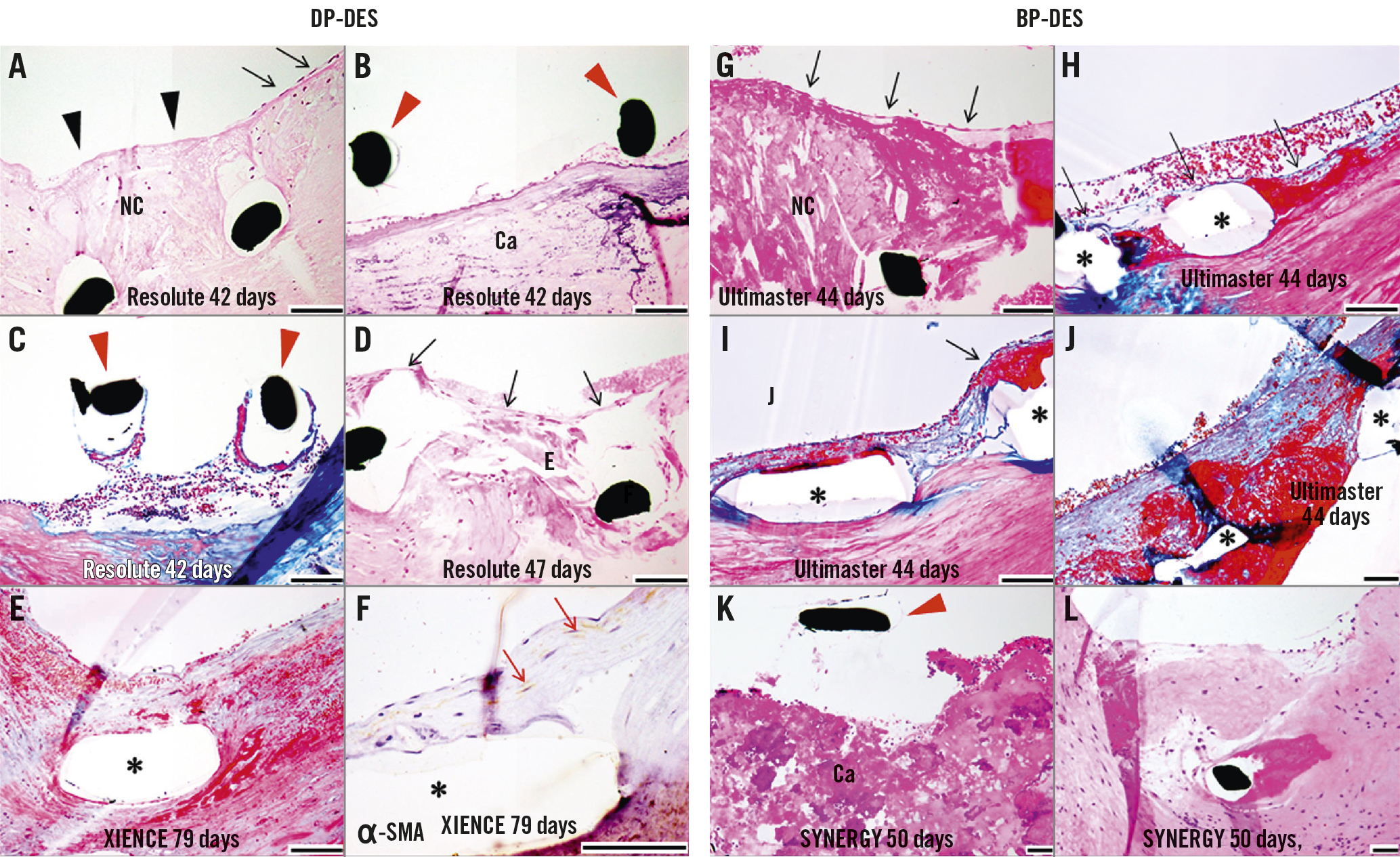
Figure 4. Representative histological images showing vascular responses to DP-DES (A-F) and BP-DES (G-L) at 1 to 3 months following the implantation. A) Struts with overlying necrotic core (NC) and absence of endothelium (black arrowheads=grade 1) along with adjacent endothelial coverage (black arrows). B) Bare struts (red arrowheads=grade 0) on the calcified plate (Ca). C) Incomplete stent apposition with partial organisation without strut coverage (grade 0). D) Single-layered endothelial coverage (grade 2). E-F) Strut is covered with endothelium and underlying smooth muscle cell (SMC) layers (grade 3, E), which is confirmed by immunostaining for ɑ-smooth muscle actin (ɑ-SMA) (red arrows, F). G) A strut with overlying NC is covered with endothelium (grade 2). H) Peristrut fibrin is observed but covered with endothelium (grade 2). I) Left strut shows grade 3 coverage and right one exhibits endothelium with underlying fibrin (grade 2, black arrow). J) Despite substantial peristrut fibrin deposition, struts are covered with endothelium and SMC layers (grade 3). K) Focal bare strut on calcified nodule (Ca) (grade 0). L) Strut with surrounding fibrin is covered with SMC layers and overlying endothelium (grade 3). Haematoxylin and eosin (A-B, D, G, K-L) and Masson’s trichrome staining (C, E, H-J). *Stent struts. Black scale bars=100 μm. BP-DES: biodegradable polymer-coated drug-eluting stent; DP-DES: durable polymer-coated drug-eluting stent
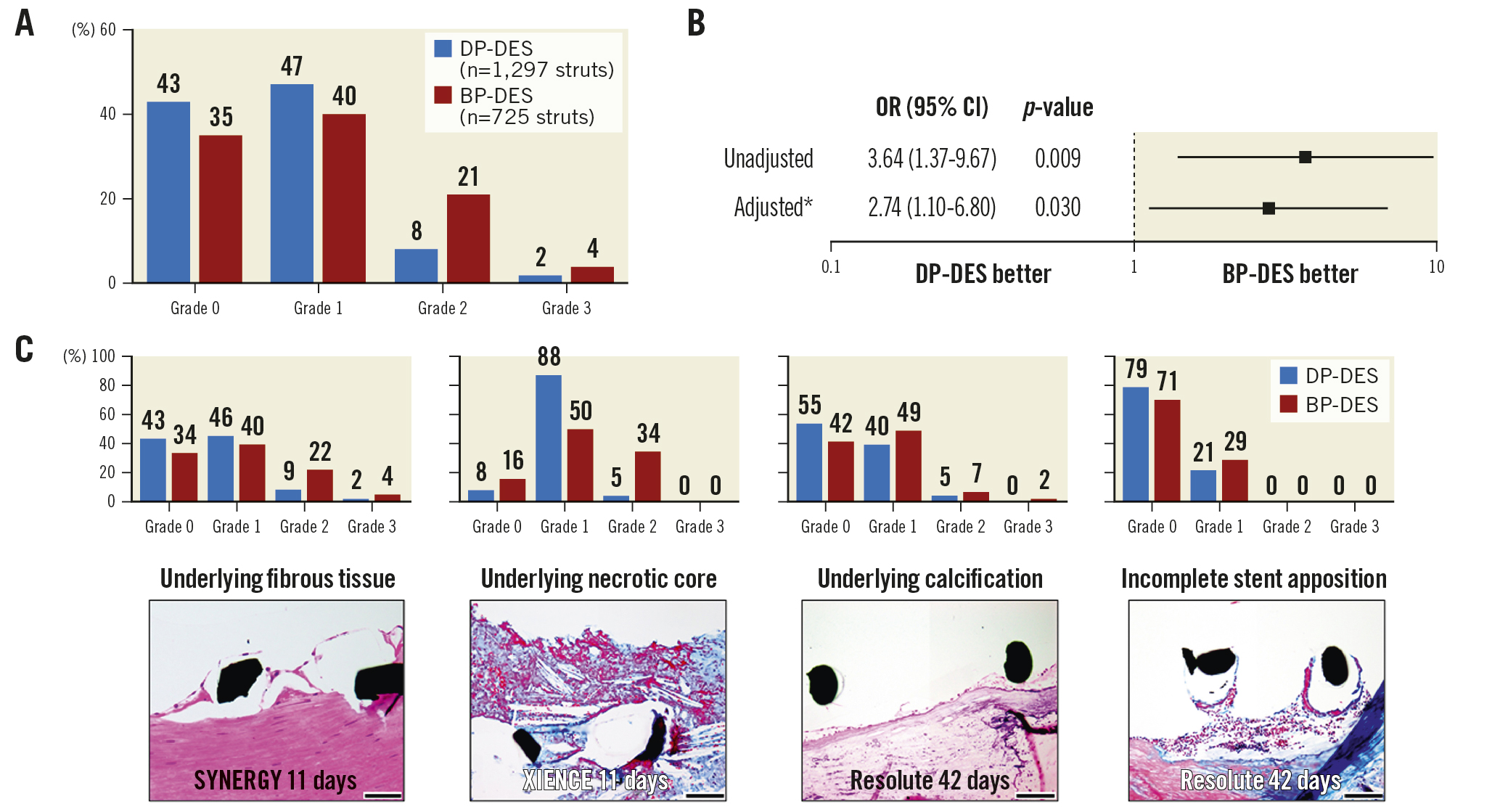
Figure 5. Comparison of the degree of strut coverage between DP-DES and BP-DES. A) The observed frequency of each degree of strut coverage in DP- and BP-DES. B) Multilevel mixed-effects ordered logistic regression model for strut coverage in BP- compared with DP-DES. C) The degree of strut coverage in various underlying tissue characteristics. The number of struts for each underlying tissue characteristic are described in Table 1. *Adjusted for duration following implantation and underlying tissue characteristics. Black scale bars=100 μm. BP-DES: biodegradable polymer-coated drug-eluting stent; CI: confidence interval; DP-DES: durable polymer-coated drug-eluting stent; OR: odds ratio
The time course of strut coverage in DP- and BP-DES
The time course of vessel healing was further assessed as the predictive probability of strut coverage (grade 0 to 3) stratified by duration following implantation using multilevel mixed-effects ordered logistic regression analysis, which revealed faster vessel healing in BP-DES compared with DP-DES (Central illustration). At 30 days, the probability of grade 2 coverage was 14.6% (DP-DES) vs 23.1% (BP-DES) and that of grade 3 was 2.5% (DP-DES) vs 5.6% (BP-DES). At 90 days, the probability of grade 2 coverage was 39.5% (DP-DES) vs 34.2% (BP-DES) and that of grade 3 was 37.0% (DP-DES) vs 52.4% (BP-DES) (Central illustration, Supplementary Table 3). Therefore, the predictive probabilities of grade 2 and 3 coverage were 17.1% (DP-DES) vs 28.7% (BP-DES) at 30 days and 76.5% (DP-DES) vs 86.6% (BP-DES) at 90 days.
Discussion
The principal findings of the current human autopsy study are as follows: (1) single-layered endothelial coverage started in the days following newer-generation DP- and BP-DES placement; (2) BP-DES exhibited greater strut coverage and achieved faster vessel healing with SMC infiltration than DP-DES even after adjustment for duration following implantation and underlying tissue characteristics; (3) the probability of endothelial coverage with or without underlying SMC layers was only 20-30% at 30 days while it increased to 75-90% at 90 days after DP- and BP-DES placement; (4) both DP- and BP-DES showed low inflammation and a similar degree of fibrin deposition in the early phase following the implantation. To our knowledge, the current study represents the first pathological assessment of the early biological responses to newer-generation DP- and BP-DES in human coronary arteries.
In recent years, DAPT for 1-3 months followed by P2Y12 receptor inhibitor monotherapy has become a novel short-term DAPT strategy12. Although shortening DAPT duration could reduce the risk of major bleeding, it could also result in a higher incidence of ischaemic events than long-term DAPT in patients with ACS89. The degree of strut coverage as assessed by optical coherence tomography (OCT) in previous clinical studies was highly variable; for DP-DES, it was reported to be 26-79% at 2 weeks and 62-98% at 1-4 months, and for BP-DES, it was 42-54% at 2 weeks and 72-92% at 1-4 months1011. However, limited resolution of OCT did not allow us to identify single-layered endothelial coverage and to differentiate thrombus, fibrin, protruding necrotic core, or other tissues from healthy strut coverage containing collagen and SMC.
In preclinical studies, several reports have shown vascular responses to BP-DES compared with DP-DES. A study in porcine coronary arteries showed greater strut coverage in BP-DES (SYNERGY) compared with DP-DES (XIENCE) at 3 days following implantation5. In an atherosclerotic rabbit iliac artery model, thin-strut BP-DES (SYNERGY) showed greater strut coverage than thick-strut BP-DES (Nobori) at 28 days and exhibited less inflammation than thick-strut BP-DES and DP-DES (Resolute Integrity) at 90 days after implantation12. Another study in a rabbit iliac artery model reported that BP-DES (Ultimaster) showed faster endothelial coverage and greater expression of endothelial barrier protein vascular endothelial cadherin as compared with DP-DES (XIENCE)13. However, it should be noted that the time course of vessel healing in preclinical models is different from that in human atherosclerotic coronary arteries containing necrotic core and calcification14. Therefore, we sought to evaluate pathological responses, in particular in the early phase of DES implantation using human autopsy cases.
Faster strut coverage in BP-DES compared with DP-DES as observed in current and previous studies may be attributed to the difference in polymer coating style and stent design (Supplementary Figure 1)5. Considering that the differences in strut coverage started to be seen in the very early phase before completing polymer biodegradation, it is plausible that the abluminal coating style, rather than the polymer material itself, encouraged faster healing. In addition, thin struts are known to be associated with greater endothelial cell coverage than thick struts15, and the strut thickness of BP-DES in the current study was indeed thinner than that of DP-DES. On the other hand, both DP- and BP-DES showed low inflammation and a similar degree of fibrin deposition in our study. Durable polymers in newer-generation DES are considered to be highly biocompatible, while the process of biodegradation of polymers may not be accompanied by such strong inflammation. The degree of fibrin deposition is known to increase with increased doses of drugs in preclinical models16. A substantial percentage of struts with fibrin deposition in the current study may simply reflect the ongoing process of drug releases in the first 3 months, where the effect of drugs seemed to be basically similar in DP- and BP-DES.
Strut coverage is the most reliable histological predictor of stent thrombosis, and <70% coverage has been shown to be associated with a markedly increased risk of events6. Healthy strut coverage is pathologically defined as luminal endothelial cells with underlying collagen and SMC layers (i.e., grade 3 coverage)17. However, single-layered endothelial coverage without apparent underlying SMC layers (grade 2) may be distinguished from bare struts (grade 0) or struts with overlying thrombus (grade 1) in terms of thromboresistance, considering the antithrombotic properties of the endothelium18. In the current study, the predictive probability of grade 3 coverage was very low, only 3-6% at 30 days and 37-52% even at 90 days. When adding grade 2 coverage to grade 3, the probability increased to 17-29% at 30 days and 77-87% at 90 days. While durable fluoropolymer coatings, but not the BioLinx polymer coating, are known to exert antithrombotic properties1920, the safety of BP-DES may be conferred by faster strut coverage. Strut coverage progressed with time to become substantial at 90 days; however, vessel healing was suboptimal at 30 days in both DP- and BP-DES, especially in cases with underlying necrotic core, which has been shown to delay vessel healing4. While the current pathological assessment showed suboptimal vessel healing at 30 days, the ideal duration of DAPT remains unclear. In the clinical setting, the duration of DAPT should be determined in individual cases, taking into account the balance between bleeding and thrombotic risk.
Study limitations
The current study involved autopsy cases who died early after stent placement mostly due to serious medical conditions, and there may be unavoidable referral and selection biases. Therefore, caution must be exercised when extrapolating the current findings to living patients. The majority of autopsy cases in this study were ACS patients and only a small number of CCS patients were included, which should be considered when interpreting the results of this study. Both DP- and BP-DES included different types of DES, and the limited number of investigated cases did not allow us to compare all different types of DES. In addition, BP-DES included only abluminal polymer-coated DES, and biological responses to circumferential BP-DES remain unknown. Single-layered endothelial coverage was assessed only by light microscopy without scanning electron microscopy, and plastic embedding did not allow us to confirm the presence of endothelial cells by immunohistochemistry. Finally, our study focused on only the early phase, where the majority of the included lesions occurred within 30 days following implantation and the time course of vessel healing up to 90 days was evaluated as a predictive probability of strut coverage. The long-term vascular responses to newer-generation DES, especially to BP-DES, remain unclear. Our stent autopsy registry had a limited number of BP-DES implanted for ≥90 days (Figure 1, Supplementary Figure 4), and future studies involving a large number of cases should clarify whether faster vessel healing in BP-DES contributes to long-term vessel health, including the prevention of in-stent neoatherosclerosis.
Conclusions
The current first human pathological study on early biological responses to newer-generation DES as assessed with accurate clinical information demonstrated that single-layered endothelial coverage begins in the days following stent placement, and abluminal BP-DES potentially exhibit greater strut coverage and achieve faster vessel healing with SMC infiltration than circumferential DP-DES. Nevertheless, the degree of strut coverage was comparably limited at 30 days in both DP- and BP-DES, and the process of vessel healing progressed with time to become substantial at 90 days following the implantation.
Impact on daily practice
The current first human pathologic study on early biological responses to newer-generation DES demonstrated that endothelial coverage begins in the days following stent placement, and abluminal BP-DES exhibit faster strut coverage with smooth muscle cell infiltration than circumferential DP-DES. Nevertheless, vessel healing remains suboptimal at 1 month in both DP- and BP-DES, and it progresses with time to reach a substantial level at 3 months following the implantation. In the clinical setting, the duration of DAPT should be determined in individual cases, taking into account the balance between bleeding and thrombotic risks, where the current findings may partly serve as a reference.
Acknowledgements
The authors thank Dr Chikao Yutani, Dr Yoshiharu Higuchi, Dr Hiroyuki Hao, Dr Takayuki Ishihara, Dr Daisuke Ito, and Dr Keisuke Ueno for their contributions to the data collection. The authors also thank Ms Mayumi Oka and all other medical staff in the department of pathology at the National Cerebral and Cardiovascular Center for their technical support.
Conflict of interest statement
F. Otsuka is supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (19K08571); and has received honoraria from Abbott Medical Japan, Amgen K.K., Bayer Yakuhin, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Kowa Pharmaceutical, Nipro Corporation, Novartis Pharma K.K., Otsuka Pharmaceutical, Pfizer Japan, Sanofi K.K., Takeda, and Terumo Corporation. Y. Asaumi has received research support from Abbott Medical Japan and Terumo Corporation. Y. Kataoka has received research support from Abbott Medical Japan; and honoraria from Nipro Corporation, Abbott Medical Japan, Kowa Pharmaceutical, Amgen K.K., Sanofi K.K., Astellas BioPharma K.K., Takeda, and Daiichi Sankyo. A. Finn has received honoraria from Abbott Vascular, Biosensors, Boston Scientific, CeloNova, Cook Medical, CSI, Lutonix Bard, SINOMED, and Terumo Corporation; and has served as a consultant to Amgen, Abbott Vascular, Boston Scientific, CeloNova, Cook Medical, Lutonix Bard, and SINOMED. R. Virmani has received honoraria from Abbott Vascular, Biosensors, Boston Scientific, CeloNova, Cook Medical, Cordis, CSI, Lutonix Bard, Medtronic, OrbusNeich Medical, CeloNova, SINO Medical-Device Technology, ReCor Medical, Terumo Corporation, W. L. Gore, and Spectranetics; and has served as a consultant for Abbott Vascular, Boston Scientific, CeloNova, Cook Medical, Cordis, CSI, Edwards Lifesciences, Lutonix Bard, Medtronic, OrbusNeich Medical, ReCor Medical, Sino Medical-Device Technology, Spectranetics, Surmodics, Terumo Corporation, W. L. Gore, and Xeltis. S. Yasuda has received research support from Terumo Corporation, Abbott Medical Japan, Bayer Yakuhin, Quintiles Translational Japan K.K., JSR Corporation, Takeda, IQVIA Services Japan K.K., and Actelion Pharmaceuticals Japan; and honoraria from Daiichi Sankyo, Abbott Medical Japan, Bristol-Myers Squibb, Takeda, Sanofi K.K., Bayer Yakuhin, and AstraZeneca K.K. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

