
Abstract
Aims: Drug-eluting stents (DES) have evolved to using bioresorbable polymers as a method of drug delivery. The impact of bioresorbable polymer on long-term neointimal formation, inflammation, and healing has not been fully characterised. This study aimed to evaluate the biological effect of polymer resorption on vascular healing and inflammation.
Methods and results: A comparative DES study was performed in the familial hypercholesterolaemic swine model of coronary stenosis. Permanent polymer DES (zotarolimus-eluting [ZES] or everolimus-eluting [EES]) were compared to bioresorbable polymer everolimus-eluting stents (BP-EES) and BMS. Post implantation in 29 swine, stents were explanted and analysed up to 180 days. Area stenosis was reduced in all DES compared to BMS at 30 days. At 180 days, BP-EES had significantly lower area stenosis than EES or ZES. Severe inflammatory activity persisted in permanent polymer DES at 180 days compared to BP-EES or BMS. Qualitative para-strut inflammation areas (graded as none to severe) were elevated but similar in all groups at 30 days, peaked at 90 days in DES compared to BMS (p<0.05) and, at 180 days, were similar between BMS and BP-EES but were significantly greater in DES.
Conclusions: BP-EES resulted in a lower net long-term reduction in neointimal formation and inflammation compared to permanent polymer DES in an animal model. Further study of the long-term neointima formation deserves study in human clinical trials.
Abbreviations
BMS: bare metal stent
BP-EES: bioabsorbable polymer-coated everolimus-eluting stent
DES: drug-eluting stent
EES: everolimus-eluting stent
NI: neointima thickness
PSI: para-strut inflammation
ZES: zotarolimus-eluting stent
Introduction
Drug-eluting stents (DES) have reduced the long-term rate of reintervention and improved clinical outcomes among patients undergoing percutaneous coronary intervention1. In large randomised controlled trials, second-generation DES improved clinical outcomes but still carried a cumulative risk of long-term target lesion failure and late stent thrombosis rates2,3. Experimental and human data suggest persistent inflammation and abnormal vessel healing as the predominant biological mechanisms of stent failure4-7 and that the continuous presence of antiproliferative drugs and/or polymers is the most important influence leading to delayed and/or abnormal strut coverage, an important risk factor for late stent thrombosis4,8. As a result, technological changes in DES have focused on reducing the impact of the polymer on these biological phenomena: next-generation DES have evolved into thinner delivery scaffolds utilising abluminal bioresorbable polymers to modulate directional drug release9. This approach aims to decrease polymer-induced vessel wall inflammation and enhance stent surface endothelialisation. This study evaluates the combined biological effect of polymer resorption on vascular healing and inflammation.
Methods
TEST ARTICLES
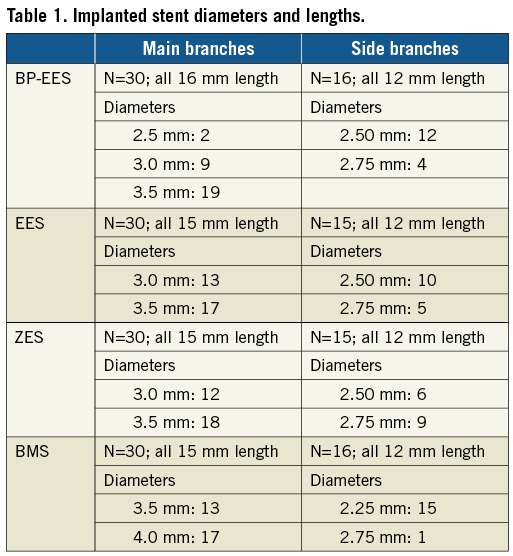
Stents tested were: 1) SYNERGY everolimus-eluting platinum-chromium stent (BP-EES) (Boston Scientific, Marlborough, MA, USA; 74 μm thick struts, 4 µm bioabsorbable poly(DL-lactide-co-glycolide) polymer applied abluminally; everolimus is eluted within 90 days and polymer resorption is complete within four months)10; 2) everolimus-eluting XIENCE PRIME® stent (EES) (Abbott Vascular, Santa Clara, CA, USA; 81 µm thick struts, 7 µm PBMA and PVDF-HFP permanent polymer); 3) zotarolimus-eluting Resolute Integrity® stent (ZES) (Medtronic, Santa Rosa, CA, USA; 91 µm thick struts, 6 µm tripolymer of C19 polymer, polyvinyl pyrrolidinone and C10 polymer permanent polymer) were studied. The bare metal stent control was the OMEGA stent (BMS) (Boston Scientific; 81 µm thick, commercialised as REBEL) (Table 1).
EXPERIMENTAL PROTOCOL
This study conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. Stent implants were performed at the Skirball Center for Cardiovascular Research, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Study procedures, including pathologic evaluation, were performed in compliance with Good Laboratory Practices as defined in the U.S. Code of Federal Regulations, 21CFR Part 58. The long-term vascular healing study was performed using the familial hypercholesterolaemic swine model of coronary stenosis11. Figure 1 shows the study design (additional information in Supplementary Table 1). Thirty swine (12-25 months old, weight 46-142 kg) were obtained from the University of Wisconsin with baseline cholesterol levels of 420±37, 443±43 and 404±75 mg/dl, for the 30, 90 and 180-day groups, respectively. Animals were maintained on a low-grade high-cholesterol diet (0.6% cholesterol; started two days before stent placement); cholesterol levels at termination were 734±144, 590±92, and 605±87 mg/dl, in the 30, 90 and 180-day groups, respectively.
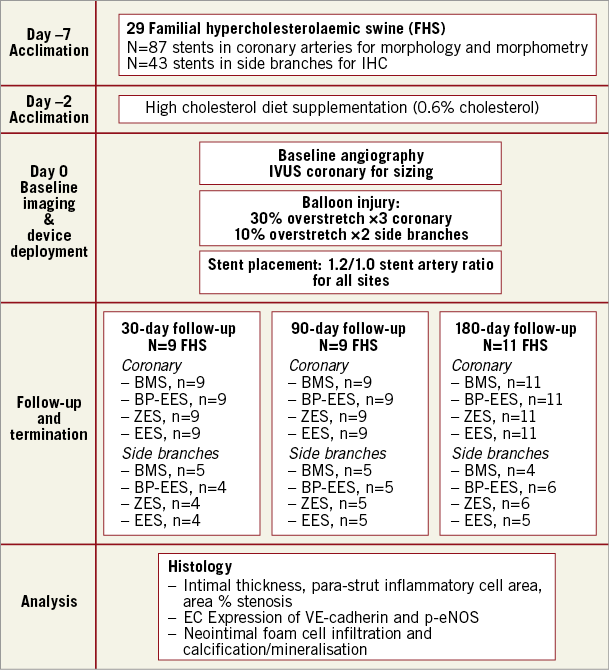
Figure 1. Overview of in vivo experimental protocol.
For balloon injury and stent implantation, animals were pre-anaesthetised with a mixture of Telazol (2-5 mg/kg) and Glycopyrrolate (0.004-0.01 mg/kg) given intramuscularly. Animals were anaesthetised by mask induction (isofluorane [3-5%/oxygen]) and maintained with inhaled isofluorane (1-3%). An activated clotting time (ACT) of >250 seconds was achieved prior to first device delivery. Animals were monitored postoperatively until fully recovered. Animals were treated with a loading dose of aspirin (325 mg) and clopidogrel (150 mg) the day before the procedure and maintained on 81 mg aspirin and 75 mg clopidogrel daily.
Main and side branch coronary artery anatomy was imaged angiographically (Supplementary Table 2, Supplementary Table 3). IVUS was performed in main coronary artery locations only at baseline for vessel-lumen measurement purposes. Balloon injury was induced before stent placement by inflating the balloon at the target site with a target balloon-to-artery ratio (BAR) of 1.3:1 (range 1.21-1.44:1) for main coronary sites and 1.1:1 (range 1.10-1.36:1) for side branch sites (Supplementary Table 4). Previous studies have shown that a 30% balloon overstretch ratio provides the best and most reproducible results12. Inflation pressure was maintained for 30 (±3) seconds and repeated three times in coronary arteries or twice in the side branches. The stent delivery system was then advanced to the pre-injured area and inflated to a target stent-to-artery ratio (SAR) of 1.2:1 (range 1.15-1.45:1). Angiographic evaluation was performed post stent deployment. All animals were implanted with each type of stent in a main coronary site (left anterior descending, left circumflex, right coronary artery) and in ≤3 side branch sites (obtuse marginals, diagonals) using a predetermined stent randomisation scheme. BMS were placed in a proximal vessel location. Side branches received 12 mm stents with diameters between 2.25 and 2.75 mm; main branches received stents 15 or 16 mm with diameters between 2.5 and 4.0 mm (Table 1). In general, the coronary artery implanted stents were used for the histopathology component of the study while the side branch implanted stents were used for immunohistochemistry, although in a few instances stents implanted in side branches were received for histopathology analysis.
At 30 (±1), 90 (±1), or 180 (±2) days following treatment, animals were euthanised and the hearts explanted for histopathological analysis. Terminal procedures were performed after anaesthetisation and final angiographic evaluation. Animals received an intravenous bolus of 10,000 units of heparin and were euthanised by qualified staff while under general anaesthesia by IV injection of a commercially available euthanasia solution.
HISTOLOGY PROTOCOL
Stented coronary artery segments were embedded in plastic and cut serially at proximal, middle, and distal in-stent regions before staining with haematoxylin and eosin and elastin trichrome. Histological sections of each stent segment were quantitatively analysed at 30, 90 and 180 days (Figure 1, Supplementary Table 1). Morphological analysis of the stented sections was performed blindly by light microscopy. For each section the lumen, external elastic lamina, and struts were traced manually and saved as individual captured feature areas. Para-strut inflammation (PSI), neointimal thickness (NI) and area % stenosis were calculated from these tracings. Intimal thickness was calculated by drawing perpendicular lines from the outside of each strut to the lumen surface; the average strut-to-lumen distance is neointimal thickness. PSI area in mm2 was defined as contiguous regions of inflammation in contact with/surrounding struts and extending into the neointima, media, or adventitia. Area percent stenosis was calculated from the traced stent profile area as follows: 100%×(1–lumen area/stent profile area). Qualitative scores for inflammation (none/minimal, mild, moderate/severe) were assigned to each section, using previously published grading criteria (Figure 2)13,14.
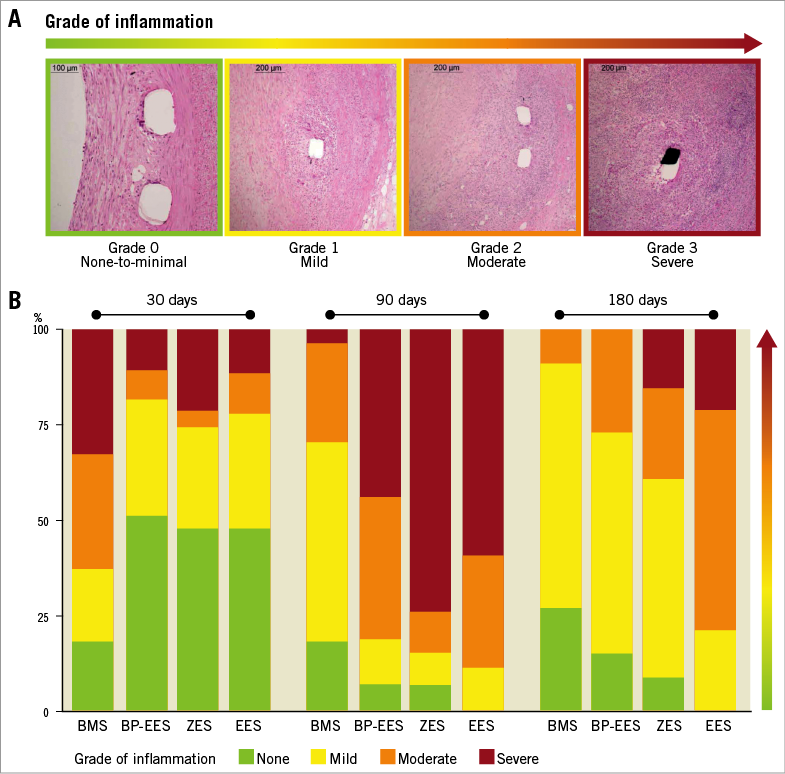
Figure 2. Visual representation of para-strut inflammation grading. A) Examples of qualitative grading. B) Para-strut inflammation scores by stent type at 30, 90, and 180 days. Combined scores for proximal, mid, and distal sections are presented as % of total sections. Green: no inflammation/grade 0; yellow: mild inflammation/grade 1; orange: moderate inflammation/grade 2; red: severe inflammation/grade 3. N=9 stents (27 sections) at 30 and 90 days; N=11 stents (33 sections) at 180 days.
Neoatherosclerosis was evaluated and graded according to Otsuka et al15. The following parameters were examined – foam cell infiltration of the neointima, media and adventitia; mineralisation/calcification; neointimal hypocellularity/necrosis; lipidic pool/cholesterol clefts; fibrous cap; haemosiderin/haemorrhage; and neointimal lipidosis (adipocytes) using the following scale: not present (0); present but a minimal feature (1); notable feature (mild) (2); prominent feature that does not disrupt tissue architecture and is not overwhelming, moderate (3); and overwhelming feature or feature that effaces or disrupts tissue architecture (severe) (4). Each cross-section of stented coronary artery was evaluated separately in four quadrants per clock face with the slide standing parallel to the stage and label to the right. The grades were tabulated by section and quadrant and then the grades were averaged for the three sections (proximal, middle and distal)15.
Sections for quantitative immunohistochemistry were cut longitudinally and stained with antibodies directed against VE-cadherin (8 µg/ml) (Santa Cruz Biotechnology, Dallas, TX, USA) or eNOS (2.5 µg/ml) (BD Biosciences, San Jose, CA, USA). Images of stented arteries en face were collected at multiple locations at 30 days (n=325 sections, 17 stents), 90 days (n=363 sections, 20 stents) and 180 days (n=383 sections, 21 stents) along the stent using a confocal microscope (60x water immersion objective). Locations along the vessel that were exposed to tissue/stent damage during necropsy or processing artefacts were excluded from analysis.
STATISTICAL ANALYSIS
PSI area in mm2, intimal thickness in mm, stent profile-based area percent stenosis, as well as eNOS and VE-cadherin expression as % coverage, were non-normally distributed. Statistical significance was determined using Kruskal-Wallis analysis of variance on ranks with multiple comparisons using Dunn’s post hoc test, and for two-group comparisons by the Wilcoxon rank-sum test. Quantitative data for all parameters (excluding neoatherosclerosis) are presented as box plots with median, Q1 (bottom of box), Q3 (top of box), as well as 5% and 95% whiskers.
For the assessment of neoatherosclerosis, only two of the nine histopathological parameters evaluated yielded detectable grades at a stent-based level as distinct from low grades (one of four) on occasional sections. Of these two, the data for intimal foam cells were normally distributed and demonstrated homogeneity of variance and could be validly analysed by ANOVA with the Bonferroni correction for multiple group comparisons. The second, calcification, was not normally distributed and differences between groups were tested non-parametrically with a Kruskal-Wallis test.
Results
Thirty animals were implanted, and 29 survived to the scheduled time point. One animal from the 180-day cohort had an unscheduled death during transport at day 32 and was not included in the analysis. No identifiable cardiovascular cause of death was found; gross necropsy found nothing significant regarding the heart, lung, or thoracic cavity.
FUNCTIONAL MARKER EXPRESSION
Evaluation of functional endothelialisation by en face examination using immunostaining indicated no significant differences in endothelial eNOS expression between stent groups at 30, 90 or 180 days (Figure 3). There was a general reduction in eNOS expression over time with reductions between 30 and 90 days and further reductions between 90 and 180 days being either statistically significant or trending towards significance (Figure 3) for all stent groups.
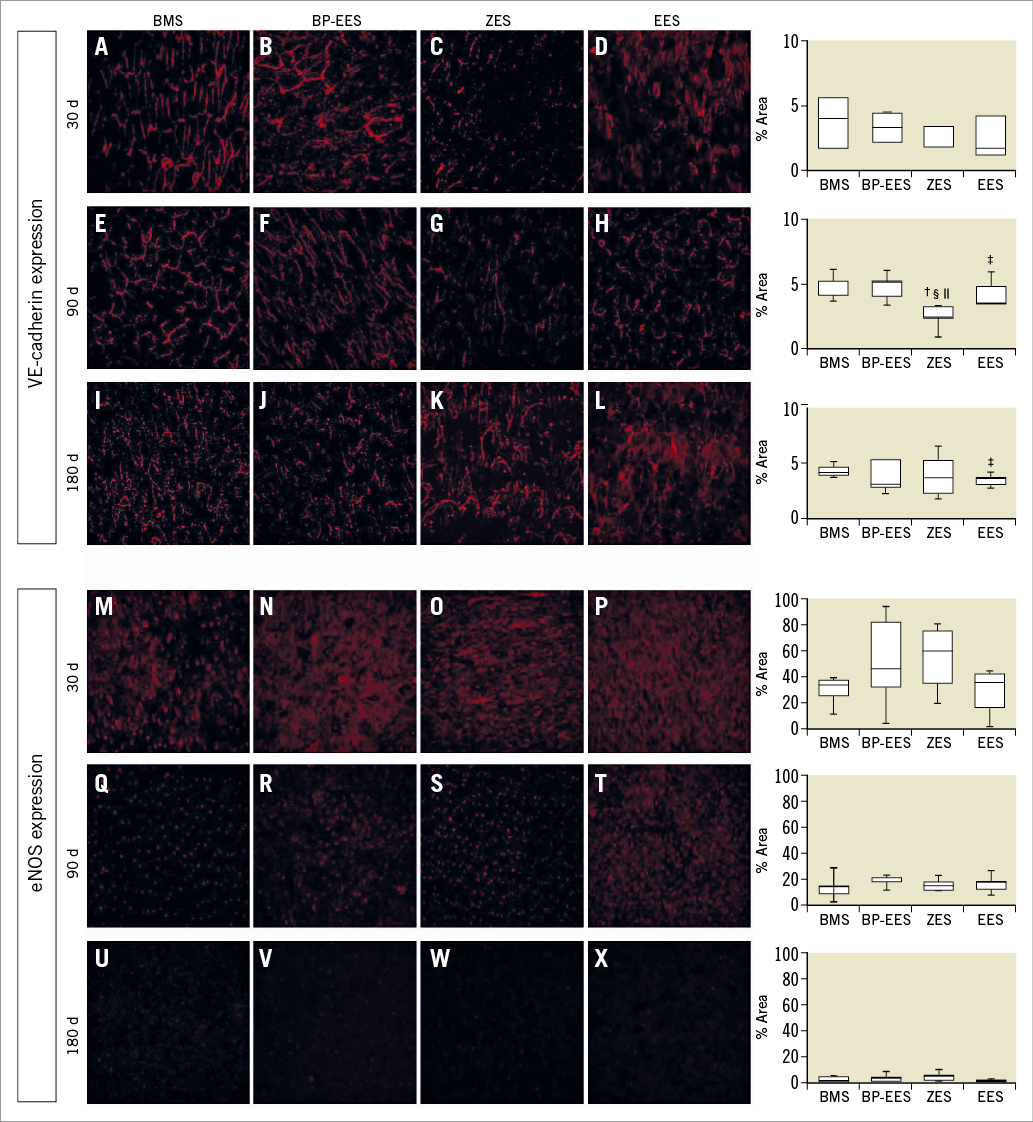
Figure 3. Expression of VE-cadherin and eNOS at 30, 90, and 180 days. Representative micrographs of VE-cadherin (A-L) and eNOS (M-X) on EC in arteries containing OMEGA, Resolute, SYNERGY, or XIENCE at all time points. Values are shown to the right as box plots with whiskers of 5% and 95%. Two-group p-values are from Wilcoxon rank-sum test; p<0.05 indicated as BMS vs. ZES†; BMS vs. EES‡; ZES vs. BP-EES§; ZES vs. EES||.
There were no significant differences in VE-cadherin expression between stent groups at 30 days (Figure 3). At 90 days, there was a significantly lower level of VE-cadherin with ZES than BMS (p=0.01), BP-EES (p=0.01) or EES (p=0.02) (Figure 3). At 180 days, EES showed significantly less (p=0.04) VE-cadherin than BMS; no significant differences between BMS and either ZES (p=0.92) or BP-EES (p=0.71) were found.
VESSEL WALL INFLAMMATION
The quantitative evaluation of the para-strut inflammatory response is presented in Figure 2. At 30 days, PSI was significantly higher (2-3x) in the BMS group, compared to all three DES groups. By 90 days, PSI had markedly subsided by ~90% of BMS sections whereas PSI levels persisted or increased between 30 and 90 days in the DES groups. At 180 days, median PSI area in BMS was significantly lower than at 30 days. PSI area at 180 days in BP-EES dropped to levels similar to BMS. PSI areas in EES at 90 and 180 days were not significantly different but were larger than BP-EES (p=0.005). PSI area for ZES was larger than BMS (p=0.04) but not BP-EES at 180 days (p=0.26).
The qualitative assessment of para-strut inflammation confirmed the patterns seen with the quantitation of PSI areas. The qualitative grades of none/no inflammation (grade 0), mild (grade 1), moderate (grade 2) and severe (grade 3) para-strut inflammation are presented as a % of sections (Figure 2). The near resolution of para-strut inflammatory activity between 30 and 90 days in the BMS was apparent and persisted at 180 days. BP-EES took longer than BMS for the inflammation to drop substantially (peak at 90 days); by 180 days there were no BP-EES sections showing severe inflammation. Though all three DES showed severe inflammation in numerous sections at 90 days, severe inflammation persisted only in the ZES and EES stent groups at 180 days.
NEOINTIMA FORMATION
The measures of intimal thickness and stent profile-based area % stenosis showed similar patterns over time (Figure 4). At 30 days, the DES groups were similar and demonstrated less intimal thickness (p<0.005 for all three comparisons) than BMS. A similar pattern was seen for stent profile area-based % stenosis. At 90 days, the differences in intimal thickness between the DES groups and BMS dissipated. Correspondingly, stent profile area % stenosis was similar between BMS, BP-EES and EES, although ZES exhibited a higher degree of stenosis than BMS. Intimal thickness with BMS did not change over time (30-180 days) (Figure 4), whereas in all DES intimal thickness rose significantly after 30 days reaching peak levels at 90 days. At 180 days, levels in ZES and BP-EES decreased from those observed at 90 days but not to the low levels seen at 30 days. With EES, median intimal thickness was maintained between 90 and 180 days. The temporal pattern of results for stent profile-based area % stenosis in the four stents was similar to intimal thickness.
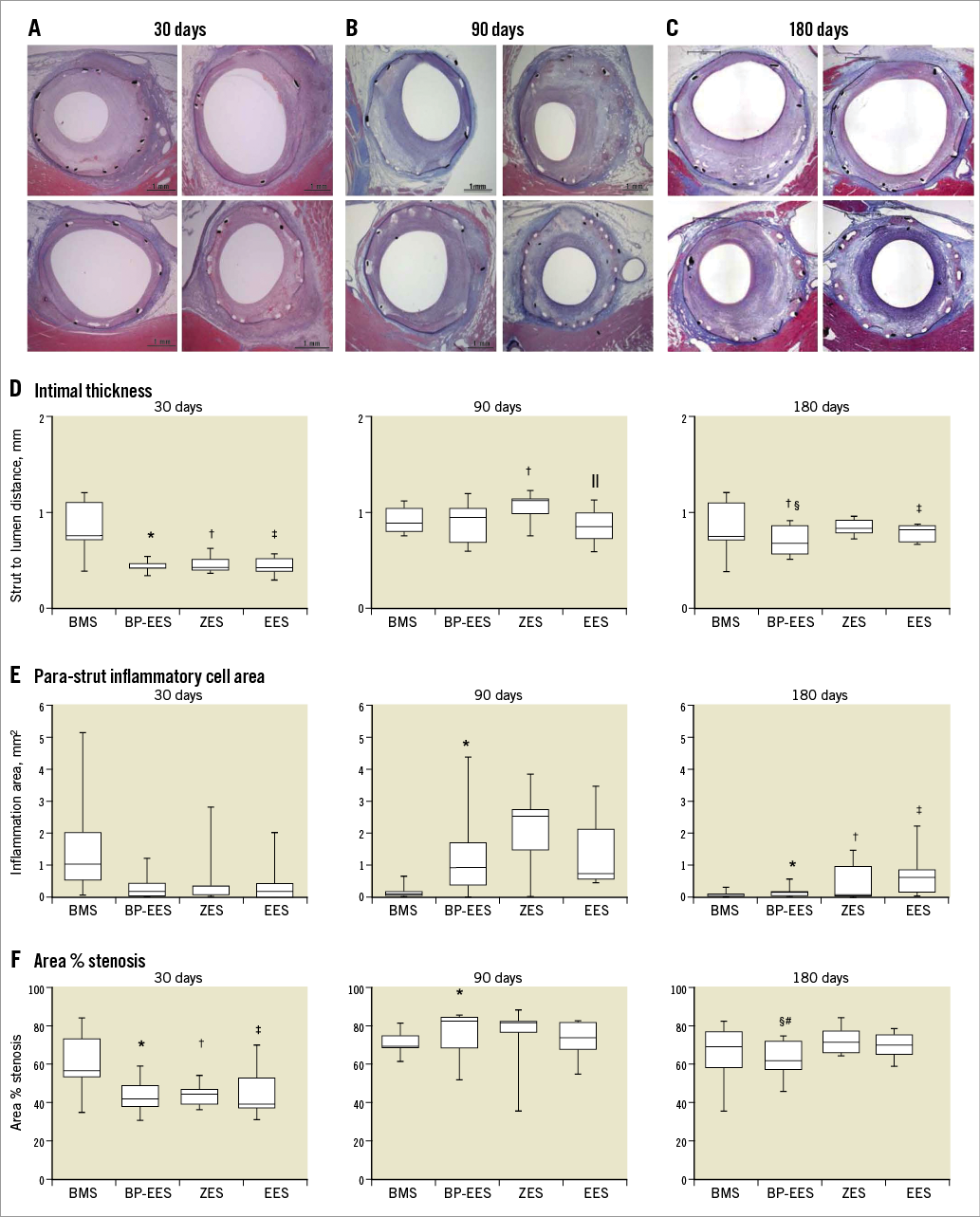
Figure 4. Progression of neointimal thickness and para-strut inflammatory cell area over time. Representative microscopic images of haematoxylin and eosin stained stent sections at 30 (A), 90 (B) and 180 (C) days. Values related to intimal thickness (D), para-strut inflammatory cell area (E), and area % stenosis (F) are shown as box plots with whiskers of 5% and 95%. Two-group p-values are from Wilcoxon rank-sum test; p<0.05 indicated as BMS vs. BP-EES*; BMS vs. ZES†; BMS vs. EES‡; ZES vs. BP-EES§; ZES vs. EES||; BP-EES vs. EES#.
NEOATHEROSCLEROSIS DEVELOPMENT
Neoatherosclerosis was evaluated at 180 days and included examination of foam cell infiltration of the neointima, media or adventitia, mineralisation/calcification, neointimal hypocellularity/necrosis, lipidic pool/cholesterol clefts, fibrous cap, haemosiderin/haemorrhage, and neointimal lipidosis. Of these nine histological parameters, only neointimal foam cells and calcification yielded scores above zero on a stent-wise basis (Figure 5). There were no significant differences in neointimal foam cell grades among any of the four stent groups. Calcification was significantly greater in each of the three DES groups than in the BMS control group but was not significantly different between any pair of the DES groups.
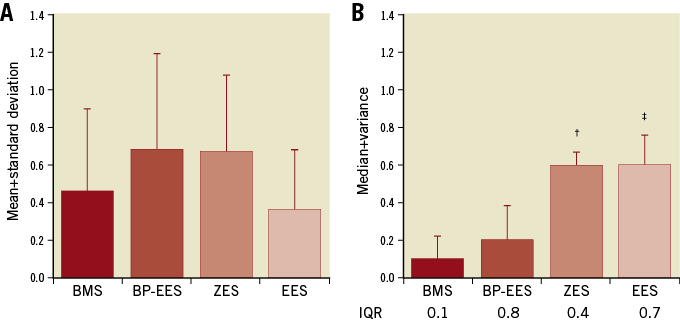
Figure 5. Neoatherosclerosis at 180 days. Scores related to neointimal foam cell infiltration (A) and calcification/mineralisation (B) at 180 days in each stent type. Grading was as follows: 0=not present; 1=present, but minimal feature; 2=notable feature, mild; 3=prominent feature that does not disrupt tissue architecture and is not overwhelming, moderate; 4=overwhelming feature or feature that effaces or disrupts tissue architecture, severe. N=11 stents per stent type graded at the proximal, mid, and distal levels of the stent. A) Data presented as mean+standard deviation; differences between groups were analysed by ANOVA with the Bonferroni correction. B) Data are presented as median+variance; differences between groups tested with a Kruskal-Wallis test. IQR: interquartile range. p<0.05 indicated as BMS vs. ZES†; BMS vs. EES‡.
Discussion
In this study, we aimed to evaluate the impact of polymer resorption following DES implantation on vessel wall inflammation and healing using a diseased swine model that displays a vascular response comparable to humans11,16. In this model, coronary implantation of a bioresorbable polymer DES resulted in lower levels of a) neointimal formation, b) para-strut inflammation and c) neointimal foam cell infiltration at 180 days compared to the permanent polymer DES tested in this study.
An important finding of our study was the difference in neointimal proliferation observed among all DES tested. The pharmacokinetic profile of durable polymer DES has been fully characterised and correlated to clinical outcomes17. Conversely, the coupling of drug release kinetics and polymer degradation in bioabsorbable polymer-eluting DES may lead to differences in pharmacokinetic behaviour and antirestenotic effect18,19. In this study, we confirmed that the antirestenotic profile of bioresorbable polymer DES was not only maintained over time but was numerically lower compared to permanent polymer DES at 180 days.
The histological evaluation of inflammatory response to coronary stents has previously been limited to the qualitative evaluation using various grading systems. In contrast, in the present study, stent-related inflammation was evaluated both quantitatively and qualitatively. PSI areas were measured by tracing the areas occupied by inflammatory cells around stent struts in each stent section. Though this approach does not distinguish between different inflammatory cell types, the inflammatory response in this study was frequently limited to a similar bland foreign body response around the stent struts with macrophages and occasional multinucleated giant cells present. In instances where PSI areas were substantially larger, the pattern of inflammatory response was a localised hypersensitivity reaction with para-strut accumulations of mixtures of granulocytes, lymphocytes and macrophages, many with multinucleated giant cell transformation, producing para-strut granulomas. Granulocytes typically included numerous eosinophils in the more intense reactions. Thus, the quantitative approach to inflammation did work well in this study (Figure 2). The qualitative grading system has been reported previously14. The results of both analytical methods indicated a remarkable similarity in the pattern of inflammatory response over time. The inflammatory profile of the BP-EES was comparable to the permanent polymer DES systems up to 90 days but dropped to low levels akin to BMS at 180 days. The two permanent polymer DES showed persistent inflammatory activity at 180 days. Overall, BP-EES demonstrated significantly lower stent-based % area of stenosis at 180 days than either EES or ZES which may be related to the well-established effect of chronic inflammation promoting neointimal hyperplasia. Both polymer and strut thickness have been shown to influence inflammation and neointima formation20. The four stents tested were between 74 µm and 91 µm with a polymer layer between 4 µm and 7 µm (no polymer on the BMS). The BP-EES used in this study was coated abluminally compared to conformal application of polymer in the two permanent polymer DES designs. Our results appear to support the hypothesis that, with absorbable polymers, in comparison to permanent polymer, long-term inflammation is decreased and healing response is promoted.
The use of en face immunohistochemistry to evaluate endothelialisation did not distinguish any substantial differences overall among the device groups. This is perhaps not surprising as the sample sizes for these side branch stents were small. The persistent decline in eNOS expression across the 30, 90 and 180-day time points for all stent types might be specific to the hypercholesterolaemic model but there is, to our knowledge, insufficient experience with this technique in various animal models to draw any conclusions. Regarding the influence of inflammatory activity on endothelial cell eNOS expression, there was no apparent difference between the low inflammation BMS control and BP-EES groups and the permanent polymer groups at 180 days. Para-strut inflammation at the time points studied, while often intense, did not extend to near the neointimal flow surface.
Our findings are also supported by another experimental study. The BP-EES used in this study has recently been examined in the atherosclerotic rabbit iliac artery model, including a comparison to ZES at 28 and 90 days21. At 28 days (in rabbits), scanning electron microscopy demonstrated that the percentage of uncovered struts was lowest with BP-EES. Foamy macrophage infiltration within the neointima at 90 days was also lowest for BP-EES. In our porcine study, the differences in neoatherosclerosis development were not striking between the DES tested. The study duration in our model did not allow time for the substantial development of this condition. However, the pattern of persistence of inflammation in permanent polymer groups at 180 days in our study suggests that polymer absorption could positively affect the natural progression of neoatherosclerosis development following DES implantation.
Limitations
Although animal experimental models have been generally accepted as useful in preclinical safety screening studies of stents, their utility for the prediction of efficacy and stent thrombotic events in humans has been limited. The hypercholesterolaemic pig was used in the present study in an effort to model healing more realistically. We acknowledge that there are limitations in the amount of coronary atherosclerosis present at the time of the implant, not comparable to the atherosclerotic changes developing over decades prior to coronary stent implantation in humans. These limitations were apparent in our evaluation of neoatherosclerosis at 180 days. Nevertheless, the pro-inflammatory nature of this model in our implementation of this study proved to be a rigorous testing environment for the DES which we compared. Furthermore, the present experimental study, with its inherent limitations, suggests that the bioresorbable polymer design approach to limiting long-term neoatherosclerosis development deserves to be examined in human clinical studies.
Conclusions
The present study investigated the impact of different DES polymer types on vessel wall inflammation and healing using a diseased animal model. This study demonstrated that the use of a bioresorbable polymer coating as a method for drug elution results in lower long-term inflammation compared to durable polymer DES. Our data suggest that this technological approach may offer biological benefits, while maintaining the beneficial antirestenotic effect of DES following the initial period of vascular injury.
| Impact on daily practice Despite the clinical success of DES in restenosis prevention, adverse stent-related events continue to occur over time. Autopsy and in vivo imaging studies suggest that chronic long-term inflammation and stent healing may contribute to these events. Different polymer types elicit different cellular responses and influence the vascular healing profile following DES implantation; bioresorbable polymers may decrease the potential impact on inflammation induced by permanent polymers and improve long-term clinical outcomes. Bioresorbable polymers result in comparable degrees of inflammation to permanent polymers during the early phase of vessel healing. In the long term, the inflammatory profile of bioresorbable polymer DES is similar to BMS. This technological approach may reduce long-term target vessel failures accrued over time leading, potentially, to reduced clinical events and reduced DAPT duration following DES implantation. |
Acknowledgements
The authors gratefully acknowledge Kristine Roy, PhD (BSC), for manuscript review and editing, and Songtao Jiang, MSc (BSC), for statistical assistance. The authors would also like to thank Shannon Kenwood, MS (BSC), for data analysis.
Funding
Boston Scientific Corporation supported this study.
Conflict of interest statement
G. Wilson was a paid consultant for Boston Scientific Corporation during this study. M. Eppihimer, N. Sushkova, B. Huibregtse and K. Dawkins are current or former employees of Boston Scientific Corporation. J. Granada has received research grants from Boston Scientific Corporation. S. Rouselle has received research grants from Boston Scientific Corporation. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix. Study procedures.
Supplementary Table 1. Schedule of study procedures.
Supplementary Table 2. Mean QCA values at treatment in main coronary arteries at baseline.
Supplementary Table 3. Mean QCA values at treatment in side branch vessels.
Supplementary Table 4. Vessel diameters by stent type using IVUS.
To read the full content of this article, please download the PDF.

