Abstract
Aims: The objective of this study was to investigate the structural integrity and early vascular response of a polylactic acid-coated (i.e., biodegradable polymer) coronary drug-eluting stent (DES) (BioMatrix™; Biosensors International, Singapore) to three currently marketed FDA/CE- mark approved non-erodible polymer-coated DES in a porcine model.
Methods and results: BioMatrix™, XIENCE V™ (Abbott Vascular, Santa Clara, CA, USA), TAXUS® Liberté™ (Boston Scientific, Natick, MA, USA), and Cypher SELECT™ (Cordis, Johnson & Johnson, Miami, FL, USA) stents were implanted in pig coronaries for seven days. Polymer integrity was assessed by scanning electron microscopy (SEM) following tissue digestion. In vitro expansion of the BioMatrix™ was also performed. SEM analysis of in vivo stents demonstrated polymer defects on the abluminal surface of all DES including polymer cracking (BioMatrix™), bridging (TAXUS Liberté™), round-small defects (Cypher SELECT™), and flaking (XIENCE V™). Histologically, the myocardium revealed no evidence of acute myocardial infarction or microscopic scarring, moreover all intramyocardial vessels were found to be patent with no evidence of emboli. In vitro results demonstrated greater BioMatrix™ polymer cracking and lifting.
Conclusions: These results illustrate the presence of polymer defects in all DES (TAXUS Liberté™, Cypher SELECT™, XIENCE V™, BioMatrix™) implanted seven-days in pigs, with absence of myocardial damage in this small number of samples. Polymer coating irregularity was greater in BioMatrix™ stent expanded in vitro as compared to in vivo, suggesting simulated benchtop deployment induces greater damage to the biodegradable polymer coating than in vivo deployment in healthy porcine coronary arteries.
Introduction
The introduction of drug-eluting stents (DES) has significantly alleviated the problem of restenosis following bare metal stent (BMS) implantation, a major limitation of percutaneous coronary intervention1-3. DES delivers antiproliferative drugs to minimise smooth muscle cell proliferation and migration, thus reducing neointimal growth. Non-erodible polymeric coatings control the delivery (rate/amount) of drugs released into surrounding tissues and into the systemic circulation of the host, however, their presence has been shown to impair vascular healing and trigger hypersensitivity reaction and inflammatory response4,5. Non-erodible polymers, utilised in first and second generation drug-eluting stents, remain in the tissue even after the drug has fully eluted. A biodegradable polymer drug coating is theorised to increase the safety of DES long term, as chronic inflammatory reaction would be reduced.
The aim of this study was to investigate the structural integrity of the biodegradable polylactic acid (PLA) polymer coating of the BioMatrix™ coronary stent (Biosensors International, Singapore) system and to determine the presence of any distal emboli in the myocardium supplied by the stented coronary artery in the porcine coronary model. The BioMatrix™ coronary stent results were also compared to three FDA/CE-mark approved non-erodible polymer DES including XIENCE V™ (Abbott Vascular, Santa Clara, CA, USA), TAXUS Liberté™ (Boston Scientific, Natick, MA, USA), and Cypher SELECT™ (Cordis Corp., Johnson & Johnson, Miami, FL, USA). In vitro (benchtop) expansion of the PLA (biodegradable) polymer-coated BioMatrix™ stent was also performed and results compared to in vivo stents.
Methodology
This study was approved by the Institutional Animal Care and Use Committee of the MedStar Research Institute and conformed to the position of the American Heart Association on use of animals in research.
Stent placement and antithrombotic regimen
Premounted BioMatrix (n=6, 3.0×15 mm), XIENCE V (n=6, 3.0×12 mm), TAXUS Liberté (n=6, 3.0×12 mm), and Cypher SELECT (n=6, 3.0×13 mm) stents were implanted in the coronary arteries of 12 pigs (Yorkshire cross-domestic swine) for a duration of seven days. Stents were deployed in pairs randomly within the RCA, and LAD or LCX by inflation to their nominal pressure for 45 sec. Following stent deployment, angiography was performed to document stent placement and vessel patency.
All animals were pretreated with aspirin (325 mg) and Plavix (75 mg) PO for three days before and on the day of implantation. Aspirin and Plavix PO were then administered daily until the day of euthanasia. Moreover, heparin (200-250 IU/kg) was administered intra-arterially before and during catherisation procedures.
Tissue harvest and processing for histology
Seven days after stenting, animals were anaesthetised and underwent coronary angiography followed by euthanasia. Three stents from each group were harvested fresh for tissue digestion and scanning electron microscopy. The remaining stented arteries were perfusion-fixed in 10% formalin, dissected from myocardium, and radiographed with a high-resolution Faxitron (Faxitron Bioptics, LLC, Lincolnshire, IL, USA). Stent samples were then embedded in methyl-methacrylate with sections taken from the proximal, middle, and distal portions of the stent and stained with haematoxylin and eosin (H&E) and an elastin (Movat pentachrome) stain.
In addition, full thickness myocardial sections distal to the stent implants were sampled circumferentially in the distribution of the major coronary arteries to include the anterior, lateral, posterior, and septal wall at three levels of the left ventricle (basal, mid, and apical). Moreover, the right ventricle was sampled at the mid and apical levels. Finally, two sections were taken around each stent. Individual slides from each myocardium section were evaluated for the presence of emboli, inflammation in response to foreign material, and myocardial fibrosis and/or infarction by a pathologist blinded as to the implant matrix.
In vitro expansion of biodegradable polymer-coated stent
Three BioMatrix stents were expanded in sterile water bath at 37°C at nominal pressure (seven ATM) for 15 seconds. The expanded stent was then air dried for 48 hours under a laminar flow hood at room temperature. The integrity of the biodegradable PLA polymer was then visualised using scanning electron microscopy.
Enzymatic digestion of tissue from the stent and scanning electron microscopy
To visualise the polymer coating, the tissue from stented arteries (n=12, three per group) was removed by enzymatic digestion6. Briefly, the stented arteries were cleaned of tissue without fixation and incubated whole in collagenase (500 IU per ml) for 48 hours at 37°C followed by pancreatin (0.5 g/100ml) for an additional 48 hours. The samples were then rinsed in de-ionised H2O, immersed in 1% Triton X-100 for four hours, and additionally rinsed in de-ionised H2O. Finally, the specimens were air dried for 48 hours and mounted on carbon planchettes and sputter-coated with gold. The specimens were visualised using a Hitachi Model S-3600N scanning electron microscope (Hitachi High-Technologies Europe GmbH, Krefeld, Germany). Regions of interest (polymer flaking, cracking, uneven coating, etc.) were photographed at incremental magnifications of ×50 and ×200 from proximal to distal regions of the stent.
SEM analysis of coating irregularity of the biodegradable polymer
To assess differences between coating irregularity of the BioMatrix biodegradable polymer in the seven-day in vivo implant and in the in vitro expanded stents, polymer defects were categorised as follows: 1) polymer coating with no defects; 2) polymer coating with cracking involving up to the full thickness of the stent strut; 3) polymer coating with cracking involving full thickness of the strut and lifting; and 4) polymer coating dislodgement. The coating defects at the strut linker positions in the proximal, middle, and distal locations of each stent were classified and counted and are reported as a percentage of total counts.
Morphological evaluation
Morphological analysis of the stented sections (proximal, middle, distal) was performed by a pathologist blinded to treatment by light microscopy using standard ordinal grading assessments for fibrin and inflammation7. A vessel injury score was determined using the method of Schwartz et al8. The presence of fibrin, giant cells, and red blood cells was evaluated for each strut and expressed as a percentage of the total number of struts in each section. Endothelial coverage was visually estimated from H&E stained slides of the proximal, mid, and distal regions of the stent and expressed as the percentage of the lumen circumference covered by endothelium.
Morphometric evaluation
Computer-assisted morphometric analysis of the in-stent sections was performed on high-resolution cross-sectional images using a combination of automated and manual techniques with calibration. The following measurements were determined for the proximal, middle, and distal sections: luminal area (mm2), external elastic lamina (EEL) area (mm2), internal elastic lamina (IEL) and stent area (mm2). Additionally, intimal thickness was measured by line measurements from the edge of the strut to the lumen boundary and averaged for each section.
Statistical analysis
Continuous variables with normal distribution were expressed as mean ±SD. Discrete variables and continuous variables with non-normal distribution were expressed as median and interquartile range (IQR). Comparisons of continuous variables with normal distribution were tested by ANOVA. A Wilcoxon rank-sum test was used to compare non-normally distributed parameters or discrete variables and the Pearson’s chi-square test for categorical variables. For categorised data, comparison was performed by the Fisher’s exact test. Normality of distribution was tested with the Wilk-Shapiro test. A value of p<0.05 was considered statistically significant.
Results
All stents were successfully deployed without dissection or thrombosis. All animals remained alive for the duration of the study.
Morphometric and histological evaluation
Histomorphometry was performed on twelve implanted coronary arteries, which included three BioMatrix, three Cypher SELECT, three XIENCE V, and three TAXUS Liberté stents. There were no statistical differences among stent groups with respect to EEL, stent and lumen areas, or neointimal thickness (Table 1). Histologically, all stents appeared widely expanded and were well apposed to the vessel wall without any evidence of luminal thrombus (Figure 1). Granulomatous inflammation was absent in all stent groups. Re-endothelialisation above stent struts was incomplete (<75%) in all groups, with no significant differences (BioMatrix: 71.78±2.04% vs. Cypher SELECT: 72.56±0.69 vs. TAXUS Liberté: 68.22±4.07% vs. XIENCE V: 71.78±2.69; p=0.27). Fibrin deposition near stent struts was greatest in Cypher SELECT as compared to the other stent groups, however these differences were non-significant (Cypher SELECT™: 81.13±4.40% vs. BioMatrix: 69.02±9.28% vs. TAXUS Liberté: 62.85±13.73% vs. XIENCE V: 64.18±16.03; p=0.28). The percentage of struts with giant cells, injury score, fibrin score, and neointimal inflammation score were also similar in all groups (Table 2). Adventitial inflammation was slightly greater in the BioMatrix and Cypher SELECT as compared to TAXUS Liberté and XIENCE V stents, however these differences were not statistically significant (BioMatrix: 1.0 [1.0-2.0] vs. Cypher SELECT : 1.0 [1.0-1.33] vs. TAXUS Liberté: 0.67 [0.33-0.67] vs. XIENCE V: 0.33 [0.33-1.33]; p=0.16).
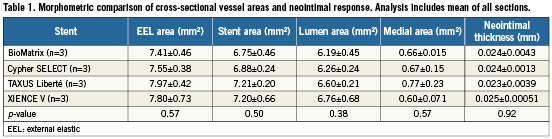
Figure 1. Histologic sections of coronary arteries seven days after stent implantation. Sections show low power (2X, bottom corner, Movat Pentachrome) and high power (200X, H&E) images of: A) BioMatrix; B) Cypher SELECT; C) TAXUS Liberté; and D) XIENCE V treated animals. In all stents, stent struts remained uncovered by neointimal growth and were surrounded by fibrin and inflammatory cells.
Myocardial findings
Overall, no myocardial abnormalities were detected. Histological evaluation of each stent group (BioMatrix, Cypher SELECT, TAXUS Liberté and XIENCE V) revealed no acute myocardial infarct, scarring, or foreign body emboli. All intramyocardial vessels were patent, with minimal inflammatory reaction (within normal limits of the model).
Enzymatic digestion of THE tissue surrounding the stent for direct visualisation of THE stent coating
The enzymatic digestion protocol successfully removed the majority of excess tissue (artery) surrounding the stents. SEM analysis revealed no loss of polymer coating from the abluminal surface of all stents, however, defects were observed in all stents (Figure 2). The BioMatrix stent coating displayed polymer cracking, mostly at the inner curve of the links. The Cypher SELECT stent had multiple round, small defects with focally uneven distribution of polymer coating. The TAXUS Liberté stent revealed polymer bridging and an area of incomplete polymer coating, exposing the stent metal. The XIENCE V stent displayed areas of uneven coating and flaking of polymer.
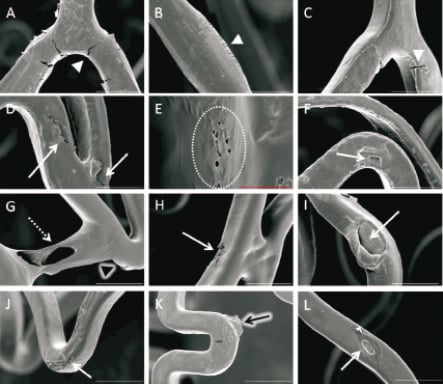
Figure 2. Representative SEM images from the enzymatic digested stents deployed into the coronary arteries of pigs for seven days. A-C) the polymer coating of the BioMatrix stent displayed polymer cracking within the inner curvature and linker bards (white arrow head); D−F)the polymer coating of the Cypher SELECT displayed uneven coating (white arrows) with small-round defects (dashed circle); G-I) the TAXUS Liberté polymer coating displayed webbing (dashed arrow), uneven coating (white arrow), and focal regions of bare metal exposure (black arrow head); J-L) the XIENCE V polymer coating displayed regions of uneven coating (white arrow) and polymer flaking (black arrow). (white bar: 200µm; red bar: 30µm)
In vitro stent expansion
The BioMatrix stent coating displayed polymer cracking and separation from the stent platform at the linker positions (Figure 3). This was consistently observed throughout the length of the stent.

Figure 3. Representative SEM images from the in vitro (benchtop) expanded BioMatrix stent in 37°C water. A) The polymer coating of the BioMatrix stent displayed polymer cracking (white arrow) and lifting (arrow head) at the curvatures; B) Within the straight linker bars, small cracks were also visible (white arrow). (white bar: 200µm)
Comparison of polymer defects between seven-day implanted and in vitro expanded biomatrix stent
A total of 36 linkers from three BioMatrix stents implanted in a pig coronary and three BioMatrix stents expanded in vitro were assessed by high power SEM images (200×) to evaluate polymer coating irregularities. Since the irregularities in the coating were observed only in the curved linkers, these were analysed in greater detail. The results demonstrated that 39% (seven of the 18) of polymer coating at the curved linkers were without coating irregularities in the seven-day implanted BioMatrix, whereas only 11% (two of the 18) of the polymer coating of the in vitro expanded BioMatrix were intact (Figure 4). Coating irregularities were observed in 61% (11 of 18) of the in vivo implanted BioMatrix stents and all displayed polymer cracking only; whereas 91% of in vitro expanded BioMatrix stents showed cracking, and of these 44% were from polymer lifting and only 6% (one of 18) was from dislodgement (Figure 4). No polymer lifting or dislodgement was observed in the in vivo samples. A Fisher’s exact test comparing results of the 36 linkers demonstrated a significant (p=0.003) difference between irregularities observed in vitro as compared to in vivo.
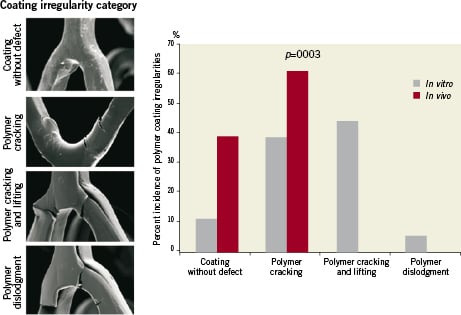
Figure 4. Comparison of coating irregularity between seven-day in vivo implanted stents and in vitro expanded stents. Coating defects were counted at the proximal, mid, and distal regions of each stent and categorised based on coating without defect, polymer cracking, polymer cracking and lifting and polymer dislodgment. Bar graph shows the differences between in vitro vs. in vivo deployment of the BioMatrix stent.
Discussion
This study demonstrated irregularities within a biodegradable polymer-coated drug-eluting stent (BioMatrix) and three current FDA/CE-mark approved non-erodible polymer coating DES, which included the Cypher SELECT, XIENCE V, and TAXUS Liberté stents implanted for seven days in healthy swine coronary arteries. The polymer coating of the BioMatrix stent displayed polymer cracking at the inner curvature of the linker positions. Polymer defects in comparator stents ranged from bridging (TAXUS Liberté), round-small defects (Cypher SELECT), rare polymer flaking (XIENCE V) and uneven polymer coating (XIENCE V, TAXUS Liberté, Cypher SELECT). Detailed gross and pathologic examination of myocardial tissue sampled distal and adjacent to implanted stents revealed no evidence of acute myocardial infarction or microscopic scarring. Moreover, all intramyocardial vessels were found to be patent with no evidence of foreign body emboli. In vitro methodology to assess the structural integrity of the abluminally-coated PLA polymer, revealed polymer cracking with lifting, and peeling. Overall, greater polymer irregularity were observed in the in vitro expanded stent as compared to the seven-day implanted BioMatrix stent.
Polymer coating of drug-eluting stents
First generation DES, Cypher and TAXUS, are covered by non-erodible polymer. The polymer coating of the Cypher stent is composed of polyethylene-co-vinyl (PEVA) and poly(n-butyl methacrylate) (PBMA), which releases 80% of the loaded dose of sirolimus in 30 days and the rest by three months1. The polymer coating of the TAXUS stent, loaded with paclitaxel, is composed of poly(styrene-β-isobutylene-β-styrene) (SIBBS), which provides a biphasic elution phase, providing a burst release lasting for two days and subsequently a low-level release over 30 days9.
It has been reported that the occurrence of a hypersensitivity vasculitis with Cypher stents is associated with extensive chronic inflammation consisting predominantly of eosinophils and T-lymphocytes infiltrating within all layers of the vessel wall, but especially marked in the peri-strut regions without any extension into non-stented segments5. This hypersensitivity reaction is reported to occur beyond the 120-day period, a time at which the drug is completely eluted from the stent as documented in animal studies carried out in normal arteries. Since the drug should be completely eluted by 90 days it is likely that the hypersensitivity reaction occurs as a result of polymer-induced inflammation. Recently, Cook et al have reported hypersensitivity in thrombus aspirates in living patients presenting with LST following Cypher stent placement and stent malapposition in both Cypher and TAXUS stents10.
Both polymers utilised in Cypher and TAXUS allow the timed release of drugs, leading to distinct elution profiles, which are necessary for antirestenotic effect. However, it is clear that non-erodible polymers can induce inflammation for a longer period since they remain intact even after the drug is completely eluted. In order to overcome these limitations it has been suggested that bio-erodible polymers might improve safety while allowing controlled release of the drug with eventual degradation, essentially leaving a bare metal surface behind, resulting in reduced long-term inflammation.
The earliest and the most commonly used biodegradable polymers are the polyesters, which include poly(lactide), poly(glycolide) and poly(glycolic-co-lactic) acids. As stated by Middleton and Tipton11, the ideal properties of biodegradable polymer should: 1) not evoke an inflammatory/toxic response; 2) be metabolised in the body after fulfilling its purpose; 3) be easily processed into the final product form; 4) have an acceptable shelf life; and 5) should be easily sterilised. Moreover, for use in stent coatings, polymer integrity is important during stent expansion and deployment, i.e., the polymer coating should remain intact with minimal irregularities during stent deployment and thereafter.
Assessment of polymer integrity: in vitro
Polymer coatings of DES control the rate and release kinetics of the incorporated antiproliferative drug that acts to minimise neointimal growth. The polymer coatings of stents are prone to mechanical damage during the deployment procedure, which includes mechanical damage incurred during negotiation of the tortuous path to deployment within the vasculature, and mechanical deformation of the coating incurred during stent expansion within diseased arteries. After deployment, thin layers of protein from circulating blood cover the polymer stent surface within minutes to hours, followed by luminal surface layering of the stent surface with platelets, fibrin, red and white cells (days to weeks), and finally the growth of neointima, consisting of smooth muscle cells and a proteoglycans matrix (weeks to months) that encapsulate the stent and polymer coating. Therefore, the majority of mechanical damage of the polymer after manufacturing occurs during the stent deployment procedure. Ultimately, the “damaged” coating is incorporated into the wall of the vessel and coated with cells and related materials through the process described above.
Studies to assess structural integrity of polymer coatings have been mostly performed in vitro. Scanning electron microscopy12-16 and/or atomic force microscopy13 are primarily used to identify any changes in polymer coating after stent expansion in vitro. SEM can easily identify polymer cracking, peeling and other irregularities, whereas AFM can measure the binding force of the polymer to the stent. For SEM analysis, stents are normally expanded in air or in 37°C water or saline, and subsequently air dried. A study performed by Basalus et al14 demonstrated polymer cracking of the Biolimus-eluting BioMatrix stents expanded in vitro. Moreover, the incidence of polymer cracking was shown to increase in BioMatrix stents expanded in 37°C water as compared to air. In our own study, we observed similar findings, as more polymer cracking was observed in BioMatrix stents expanded in 37°C water as compared to air (data not shown). Subsequently, the incidence of polymer cracking was reduced if the stent was expanded within silicone tubing with similar geometry to and compliance with coronary arteries, coated with 7.5% BSA (data not shown). The silicone tubing seemed to reduce lifting and displacement of the polymer coating, particularly as the polymer is only coated abluminally on the BioMatrix stent. These results suggest that the methodology used in in vitro models directly impact polymer coating integrity results.
Although many in vitro studies have provided valuable information, the assessment of polymer coating integrity in vitro remains somewhat controversial, as no current benchtop model can accurately mimic native arterial conditions. Currently, FDA regulations recommend coating integrity of DES to be assessed by visual techniques (SEM) post-expansion in air to establish baseline comparison to other methodology (www.FDA.gov). Moreover, it is recommended that DES be deployed within in vitro models mimicking in vivo physiologic and anatomic conditions. These experiments are intended to demonstrate that the polymer coating does not significantly change in configuration or prematurely delaminate from the stent substrate upon expansion or deployment. However, the description of “significant” changes in polymer coating still remains undefined, and will strongly depend on the polymer material and coating techniques used. It is our contention that new and novel in vitro models using test methods replicating native arteries, with physiological parameters of flow and cyclic pressure, are needed to accurately represent clinical conditions.
Assessment of polymer integrity: in vivo
In vivo evaluation of the polymer coating integrity of implanted DES has not, to our knowledge, been investigated by direct visualisation using SEM. Kitahara et al17 have shown damage to the polymer coating of an undelivered TAXUS stent as examined by SEM. The polymer of the TAXUS stent was shown to be dislodged, with exposure of bare metal stent. In our study, we successfully digested the surrounding tissue of three FDA/CE-mark approved non-erodible polymeric DES (Cypher, TAXUS, XIENCE V), as well as a biodegradable polymeric DES (BioMatrix) implanted in swine coronary for seven days. The study duration (one week) was selected, as our interest was to analyse acutely the polymer coating integrity and to ensure that any adverse effect from stent and polymer coating, such as foreign body emboli and myocardial infarction, could be identified. Moreover, with longer durations, our ability to digest tissue around the stents becomes difficult and the erodible coatings begin to lose mass and strength.
SEM analysis of the abluminal side of the polymer coating of the seven-day implanted stents revealed irregularities of the polymer in all stents. In the three FDA/CE-mark approved non-erodible polymer DES, uneven coating of polymer was observed with other polymer defects including webbing (TAXUS Liberté), and focal regions of exposed bare struts (TAXUS Liberté, Cypher SELECT, XIENCE V). Similar irregularities in the Cypher, TAXUS, and XIENCE V have been demonstrated by others using in vitro studies15. For the bio-erodible polymer-coated stent (BioMatrix), the irregularities were mostly polymer cracking observed at the linker positions. As observed by Basalus14, cracks within the BioMatrix polymer during expansion are most likely related to the low elasticity of the PLA. Interestingly, only cracking of the polymer coating of the implanted BioMatrix was observed, whereas polymer cracking with lifting and dislodgment was observed in the in vitro expanded stent. Therefore this suggests that traditional in vitro testing methods of polymer coating, which include deployment of stents in 37°C saline/water or air, may not be representative of in vivo behaviour, particularly in biodegradable coated stents such as the BioMatrix stent, which is only abluminally coated.
The patho-physiological (cardiac) impact of polymer coating damage in DES is not very well understood, as current non-invasive (CT, MRI) and minimally invasive techniques (IVUS, OCT) cannot detect structural damage and subsequent polymer coating embolic material during and after stent deployment. To overcome these limitations, myocardium sections in the pig model distal to the stent were extensively investigated by light microscopy to identify any foreign body embolic material within the intramyocardial vessels as well as areas of myocardial necrosis. Although polymer defects were demonstrated in all DES, no cardiac event (including emboli, intra-coronary occlusions, inflammation, and myocardial necrosis or infarction) was observed. Therefore, this study suggests that polymer defects observed in this study may not lead to any coating embolisation resulting in any acute cardiac event. However, the influence of uneven polymer coating and flaking, and webbing may result in an uneven distribution of drugs and lead to either rapid or further delay in healing of stented arteries. However, other factors such as thrombus, flow environment or underlying plaque characteristics have been shown to influence vessel drug interaction18. The BioMatrix stent, at least in our study, was associated with greater inflammation in the adventitia, although not significantly different from non-erodable polymers. Similarly, Kao et al showed that cracking is associated with the greater macrophage giant cell reaction19. Long-term studies are needed to determine the influence of biodegradable polymer coating disruption and simultaneous degradation on chronic inflammation and healing.
In the three-year clinical follow-up of the LEADERS trial, a prospective, randomised non-interiority trial comparing 857 Biolimus-eluting stent with biodegradable polymer (BES) and 850 patients treated with and Sirolimus-eluting stent with durable polymer (SES), the data presented at TCT 2010 showed an equivalency in the overall safety of the BES as compared to SES with similar rates in cardiac death (BES: 4.2% vs. SES: 5.2%; p=0.34), MI (BES: 7.1% vs. SES: 7.2%; p=0.97), TVR (BES: 9.4% vs. SES: 11.1%; p=0.25), and MACE (BES: 15.7% vs. SES: 19.0%; p=0.09). In the sub-analyses, focusing on treatment of bifurcation lesions within the LEADERS trial at one year, Garg et al demonstrated a significant reduction of all TLR (BES: 4.7% vs. SES: 12.1%; p=0.003) and all TVR (BES: 6.2% vs. SES: 14.2%; p=0.004) using BES as compared to SES, with similar rates in cardiac death (BES: 2.7% vs. SES: 2.9%; p=1.00) and MACE (BES: 12.8% vs. SES: 16.3%; p=0.31)20. For myocardial infarction, results were also found to be similar, however, the incidence of MI was greater in BES as compared to SES (BES: 8.9% vs. SES: 5.4%; p=0.17). This increase was driven by the significantly higher incidence of periprocedural MI (0-2 days; p=0.03). This increase, as pointed out by the authors, maybe relate to the significant greater lesion pre-dilatation performed in the BES versus SES (88% vs. 43%; p=0.03). Moreover, there was a greater number of true bifurcations in the BES group as compared to SES (BES: 131vs. SES: 102). Taking into consideration our study, we speculate that in vivo polymer cracking likely did not contribute to the periprocedural MI increase noted in the BES stent group and the increase of the periprocedural MI may be due to technique or the severity of the treated plaque. However, preclinical studies specifically designed to investigate stenting at bifurcations, both for durable and biodegradable polymer DES, will ultimately provide better understanding.
Study limitations
The stents in this study were deployed in normal arteries, therefore the results may not be representative of human atherosclerotic disease. Diseased arteries may induce more damage to all DES with polymeric coatings, however this is yet to be proven. Moreover, stents were deployed to their nominal size without any overlapping or side branch expansion, which may not represent all clinical settings. Although the myocardium assessment was performed in a total of twenty-two sections per heart, it is possible that a few occluded intra-coronary arteries associated with polymer emboli may have been missed. The enzymatic digestion process may have resulted in additional irregularity of the polymer coating. Polymer integrity was only assessed away from stent edges. For our in vitro experiment, additional irregularity from manipulations may have occurred during removal of the stent from the balloon following dilation. Although three stents per group were sufficient to show differences between groups, larger number samples would increase the significance of the data.
Conclusions
These results demonstrate the presence of surface defects in commercially available (FDA or CE-mark approved) stents (TAXUS Liberté, Cypher SELECT, XIENCE V, BioMatrix) implanted for seven-days in healthy swine. Although limited in the number of samples (n=3 per group), histomorphometric evaluation of stented arteries revealed no differences in inflammation, injury, fibrin, endothelialisation and intimal thickness in all groups. Myocardium analysis revealed an absence of adverse myocardial defects from embolisation of the coatings. In vitro methodology used to assess the structural integrity of the abluminal coated PLA polymer stent did not appropriately simulate deployment in native healthy porcine coronary arteries, as greater cracking and peeling of polymer was observed in bench testing as compared to in vivo implanted stents.
Conflict of interest statement
Dr. Renu Virmani receives research support from Medtronic AVE, Abbott Vascular, Atrium Medical, OrbusNeich Medical, Terumo Corporation, Cordis Corporation, Biosensors International, Biotronik, and Alchimedics; and is a consultant for Medtronic AVE, Abbott Vascular, W.L. Gore, Atrium Medical, and Lutonix. Dr. Shih-Horng Su is a salaried employee of Biosensors International. The remaining authors have no conflict of interest to declare.

