
Polymeric materials have long been used in the vasculature1,2. We know that both degradable and permanent polymers can elicit intense inflammatory responses in the arterial wall, even if the polymer is deemed inert3. Much knowledge has been obtained from studies searching for suitable materials to create synthetic vascular grafts. We have learned that catalysts, initiators, polymer degradation products and contaminants could all elicit inflammatory responses, even when present in small amounts (parts per million). Importantly, inert polymers could even elicit intense inflammatory responses years after implantation, when disintegrating into small particulate matter3. More than 20 years ago, a multicentre preclinical trial tested a number of polymers to study their applicability for the vascular bed4. This study marked the beginning of the era of drug-eluting stents (DES) and was a first attempt to discover which polymers might be suitable as coatings or replacements for metallic stents. DES studies have taught us that the inflammatory and neointimal response is not necessarily dictated by (degradation of) the polymer alone, but rather by the balance between drug release and polymers, degradants and the like5. We have learned that the irritant effects of polymers leading to an increase in neointimal thickening could suddenly become apparent. For instance, when drug levels to suppress proliferation dive below the suppressant threshold concentration of the drug, this could lead to neointimal catch-up5. How the extent of atherosclerosis alters the vascular response to polymeric stents and coatings remains largely unknown.
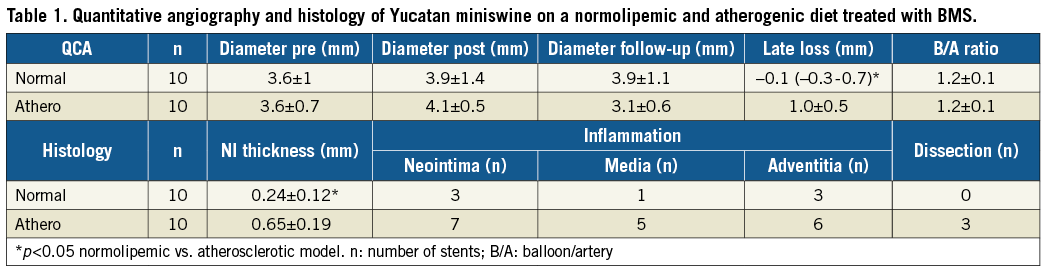
What we do know is that atherosclerosis alters the vascular response to stents with a clear relationship between plaque mass and neointimal hyperplasia after stenting6,7. Unpublished data from our group (Table 1) support this. In this study, Yucatan miniswine were placed on a normal or a high cholesterol diet6, and bare metal stents (BMS) were placed as guided by quantitative coronary angiography (QCA). At six-week follow-up, animals were sacrificed and stents were processed for routine histology5, as previously described. Data showed a 2-3x larger neointimal thickness and area after stenting for animals on the atherogenic diet. Atherosclerosis (or the atherogenic diet) also increased inflammation and susceptibility to dissection. Clearly, the data show an effect of atherosclerosis on vascular healing for these polymer-free stent systems, and the evidence is building that the response to polymers is also affected.
Indeed, we found an altered response to the Absorb™ bioresorbable scaffold (Abbott Vascular, Santa Clara, CA, USA) when studied in a setting of atherosclerosis, not only in terms of intimal lipid accumulation but as intense strut and intimal calcification that was not previously observed8-10. Given the evidence above, studying the response to DES and polymers in a setting of pro-inflammatory atherosclerosis is therefore of importance for preclinical studies to increase their predictive value.
In this issue of EuroIntervention, Wilson and colleagues in their paper “Impact of bioresorbable versus permanent polymer on long-term vessel wall inflammation and healing: a comparative drug-eluting stent experimental study” have done just that11.
They report the use of an atherosclerotic swine coronary injury model to study long-term biocompatibility of degradable and permanent polymers. They compare a BMS with permanent polymer and bioresorbable polymer DES up to six months after implantation in animals suffering familial hyperlipidaemia and placed on a high cholesterol diet, albeit only two days prior to the procedure. Their main finding pertains to the differences in neointimal proliferation and inflammation among the tested stents in their animal model. All DES show efficacy in reducing intimal thickening at 30 days versus BMS, with a catch-up at 90 days, at which time the response to the stents becomes very similar. Inflammation, while suppressed at 30 days in all DES versus BMS, numerically increases in all DES at 90 days as compared to BMS where inflammation virtually disappears. At 180 days, all DES show a numerical decrease in inflammation but least so in the permanent polymer DES, which is of importance as it may affect the vasculature and risk of complications. Interestingly, however, this seems not to have translated to changes in expression of VE-cadherin and eNOS. Another surprising finding is that regression of intimal thickening was not observed in any of the stents at 180 days, either in DES or in BMS. This is in contrast to studies in non-atherosclerotic animal models where regression can be observed, a phenomenon also observed in clinical studies5,12. Whether this is related to insufficient follow-up or to studying the vascular response against a background of atherosclerosis rather than in healthy models remains to be determined. What is certain is that implementing atherosclerotic models is a first step to implementing more clinically relevant animal models.
Conflict of interest statement
The authors have no conflicts of interest to declare.

