Abstract
Aims: We aimed to compare the vascular effects exclusive to antiproliferative agents by using identical stent and biodegradable polymeric matrices eluting everolimus (BP-EES) (Carlo; Balton) and paclitaxel (BP-PES) (Luc-Chopin2; Balton) in the porcine model of coronary injury.
Methods and results: A total of 37 stents were implanted with 110% overstretch in the coronary arteries of 14 domestic pigs: 13 BP-PES, 16 BP-EES and eight bare metal stents (BMS) (Chopin2; Balton). Coronary angiography was performed after 28 and 90 days, the animals were sacrificed and the stented segments harvested for histopathological evaluation. At 28 days, BP-PES most effectively limited angiographic late loss (LL PES: 0.15±0.1 vs. EES: 0.40±0.3 vs. BMS: 0.5±0.2 mm; p=0.04) and neointimal thickness (NT) in histology (PES: 0.12 [0.1-0.2] vs. EES: 0.38 [0.3-0.4] vs. BMS: 0.35 [0.3-0.4] mm; p<0.01). The BP-PES had lower endothelialisation (EES: 100% vs. PES: 40±4% vs. BMS: 97.5±5%; p<0.01) and slightly higher inflammation scores (EES: 1 vs. PES: 2.1±0.3 vs. BMS: 1; p<0.01). At three months, LL remained unchanged in the EES and BMS groups in contrast to an increase in the PES group (EES: 0.38±0.3 vs. PES: 0.52±0.4 vs. BMS: 0.51±0.3 mm; p=0.69). NT stabilised at 90 days in the EES group in comparison to a fourfold increase in the PES group and a 30% increase in the BMS group (EES: 0.35 [0.3-0.5] vs. PES: 0.53 [0.5-0.8] vs. BMS: 0.46 [0.4-0.5] mm: p=0.07). Stent endothelialisation and inflammation were comparable at 90 days in all groups.
Conclusions: Temporal differences in vascular response were seen by the delivery of different antiproliferative agents. In contrast to everolimus, paclitaxel seems to induce a slightly higher degree of inflammation in the short term, potentially leading to further neointimal hyperplasia in the long term.
Abbreviations
BMS: bare metal stent
BP-EES: biodegradable polymer everolimus-eluting stent
BP-PES: biodegradable polymer paclitaxel-eluting stent
DES: drug-eluting stent
EEL: external elastic lamina
IEL: internal elastic lamina
LL: late lumen loss
%DS: percent diameter stenosis
%AS: percent area stenosis
Introduction
Second-generation drug-eluting stents (DES) have proved their advantage over first-generation paclitaxel- or sirolimus-eluting DES in terms of a reduction in major adverse cardiac events, repeated revascularisations and stent thromboses1-4. These novel stents incorporated new thin-strut cobalt-chromium stent platforms, more biocompatible or biodegradable polymer matrices and new rapamycin analogues, like zotarolimus and everolimus, with controllable drug release kinetics. Several clinical studies have shown differences in adverse events among patients receiving different DES platforms5-7. Although some clinical trials8 and early preclinical investigations3,9 have hypothesised the role of paclitaxel in the overall outcome and biological effect, there are limited data studying the independent role of an eluted drug in the overall vascular healing response. Therefore, in this study we aimed to assess the vascular effects exclusive to the type of antiproliferative drug eluted, via identical biodegradable polymer matrix and stent platforms in the porcine model of coronary injury.
Methods
DEVICE DESCRIPTION
All stents used in this study utilised the Chopin® BMS, 316L stainless steel, closed-cell, 120 μm strut platform (Balton Ltd, Warsaw, Poland). Both tested and reference stents are covered with a multilayer structure containing a fully biodegradable copolymer of polylactic and glycolic acid. The total mass of the polymer on a 3.0×15 mm stent does not exceed 360 µg. In vitro evaluations show that the coating degrades entirely in eight weeks. To inhibit neointimal hyperplasia, the studied stent (BP-EES, Carlo; Balton) employs everolimus at a dose of 1.0 µg/mm2, while the reference stent (BP-PES, Luc-Chopin2; Balton) employs paclitaxel at an identical dose. All stents utilised in this study were 3.0 and 3.5 mm in diameter and 18 mm in length.
STUDY DESIGN
A study flow chart is presented in Figure 1. A total of 14 domestic swine (DS) of both genders were included. All animals ranged from five to seven months old with an average weight of around 45 kg at the time of enrolment. Middle arterial segments, without side branches of all three coronary arteries (RCA, LAD, LCX), and with appropriate diameters to ensure a 110% overstretch, were screened for stent implantation. After QCA evaluation, 37 segments were eligible for the study. After randomisation in 2:2:1 fashion, a total of 16 BP-EES (study group), 13 BP-PES (reference group) and eight BMS controls were implanted after live quantitative coronary angiography (QCA) guidance. Additional inflations were performed based on the target site diameter. Half of the animals were followed up for 28 days and the other half for three months. Subsequently, control coronary angiography was performed and the swine were sacrificed. All arterial segments were dissected and harvested for pathological and histomorphometric analysis. All interventions and analyses were blinded to operators and investigators.
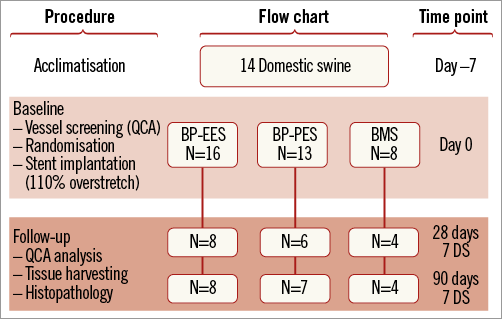
Figure 1. Figure depicting the study design flow. BMS: bare metal stent; BP: biodegradable polymer; DS: domestic swine; EES: everolimus-eluting stent; PES: paclitaxel-eluting stent; QCA: quantitative coronary angiography
EXPERIMENTAL PROCEDURES
The study protocol was approved by the local ethics committee for animal research. All animals received standard care outlined in the study protocol and in accordance with the Act of Animal Welfare and the “Principles of Care of Laboratory Animals”10.
Three days prior to the procedure, dual antiplatelet therapy consisting of 75 mg of clopidogrel and aspirin per day was initiated and continued until termination. All pigs were fasted overnight before the stent implant procedure. Animals were premedicated with atropine (0.5 mg) and subsequently sedated with intramuscular ketamine hydrochloride (20 mg/kg) and xylazine (2 mg/kg), intubated, and anaesthetised with an intravenous propofol bolus (20-40 mg) followed by a continuous infusion (2-4 mg/kg/h). Electrocardiogram readings and blood pressure were continuously monitored. A vascular sheath (6 Fr) was placed in the right or left femoral artery utilising the Seldinger technique. Anticoagulation with heparin was achieved (3,000-10,000 U) to maintain a coagulation time ≥250 seconds. Following coronary angiography, all coronary vessels were sized for proper stent implantation after live QCA analysis.
All pigs were anaesthetised and prepared in the same fashion (as described above) at 28 days and three months following stent implantation in order to perform control coronary angiography. Subsequently, they were humanely sacrificed with a pentobarbital overdose.
QUANTITATIVE CORONARY ANALYSIS (QCA)
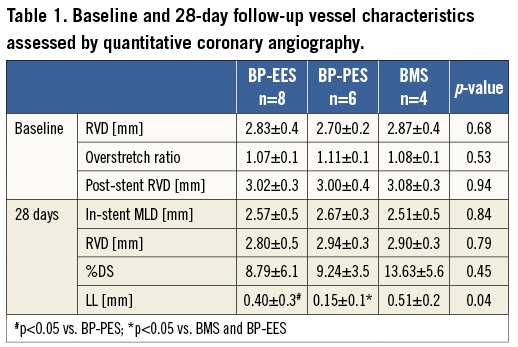
Coronary artery angiographies were obtained using the Siemens Coroskop Millennium Edition angiographic unit (Siemens AG, Munich, Germany). A Judkins right, 6 Fr guiding catheter was utilised for coronary angiography and stent implantation. QCA analysis was performed in a blinded fashion utilising QAngio XA Software version 7.1.14.0 (Medis Medical Imaging Systems, Leiden, The Netherlands) from two contralateral projections. The baseline and 28-day follow-up reference vessel diameters (RVD) were taken from the proximal and distal portions of the treated segments using the guiding catheter as a standard for measurement. The balloon-to-artery ratio was calculated. Percent diameter stenosis (%DS) at follow-up was calculated as: (1-[MLD/RVD])×100%.
HISTOLOGICAL ANALYSIS
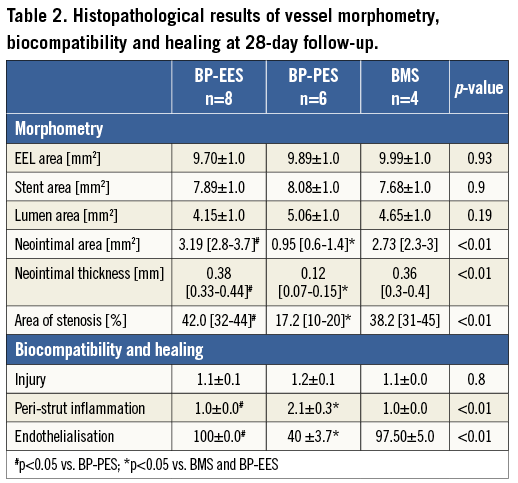
Following vessel harvesting, stented segments were immersed in normal buffered formalin 10% (NBF). For light microscopy, all treated vessels were embedded in methylmethacrylate and then 40-50 micron sections from the proximal, mid and distal portions of each stented segment were obtained. These sections were stained with haematoxylin and eosin (H&E). The cross-sectional areas (external elastic lamina [EEL], internal elastic lamina [IEL] and lumen area) of each section were measured utilising Cell^B software (Olympus BioSystems). Neointimal thickness was measured as the distance from the inner surface of the stent struts to the luminal border. The following measures were then used to calculate vessel layer areas: media=EEL-IEL; neointima=IEL-lumen; % area stenosis=[1- (lumen area/IEL area)]×100. All sections were evaluated using semi-quantitative scoring criteria. To evaluate the amount of injury, the criteria defined by Schwartz et al11 were utilised: 0=IEL intact, 1=IEL lacerated, 2=media lacerated, 3=EEL lacerated. To evaluate the extent of peri-strut inflammatory reaction the following grade by Kornowski et al12 was used: 0=minimal inflammatory response around strut, 1=few inflammatory cells around strut, 2=mild to moderate inflammation, can extend into but does not efface surrounding tissue, 3=dense and thick peri-strut aggregate of inflammatory cells, effacing surrounding tissue. Each strut in the section was scored and the mean inflammation and injury score for each section was calculated and reported.
STATISTICAL ANALYSIS
Normally distributed parametric data are expressed as average and standard deviation, and as median and interquartile range [IQR] in cases of skewed distribution. When equal variance and normality were observed, one-way analysis of variance (ANOVA) with Student-Newman-Keuls post hoc ANOVA tests were used to test for differences in variables among stent types. When either the equal variance test or the normality test failed, the Kruskal-Wallis test (with Dunn’s method for post hoc group comparison) was conducted. A value of p≤0.05 was considered statistically significant.
Results
SHORT-TERM FOLLOW-UP
In a group of animals assigned for 28 days follow-up there were no differences in baseline vessel diameters and overstretch ratios (Table 1). In the QCA analysis the late lumen loss was decreased by 21.6% in the BP-EES and 72.5% in the BP-PES groups when compared to the BMS group (p<0.05 for PES vs. BMS). In the histological analysis (Table 2), the amount of injury was comparable among groups. The measures of neointimal hyperplasia (neointimal thickness, neointimal area and percent area stenosis) in BP-PES were decreased on average by 62% when compared to BMS (p<0.01), whereas there were no differences between BP-EES and BMS as presented in representative cross-sections in Figure 2. The significant inhibition of neointimal hyperplasia came at a cost of moderate inflammation and lower endothelialisation when compared to BP-EES and BMS (Figure 3).
LONG-TERM FOLLOW-UP
The result of quantitative coronary angiographic analysis is presented in Table 3. In the group of animals assigned for 90-day follow-up, the baseline vessel diameters in the paclitaxel group were statistically larger; however, numerically the differences were modest. There were no differences in terms of overstretch ratios and post-stent implant vessel diameters. The late lumen loss was lowest in the everolimus-eluting stent group, whereas it was similar between BP-PES and BMS, although this difference was not statistically significant (p=0.57). In histopathology (Table 4), neointimal area and thickness were reduced in BP-EES by 36% and 34%, respectively, when compared to BP-PES (p<0.05 and p=0.07), and by 15% and 24% when compared to BMS (p=ns). Percent area stenosis was reduced on average by 30% when compared to BP-PES and BMS (p=0.18) (Figure 2). All tested stents showed a high level of biocompatibility as expressed by low inflammatory scores (Figure 3D- Figure 3F). Endothelialisation was also complete in all specimens.
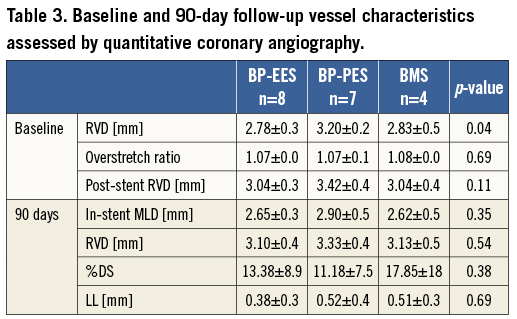
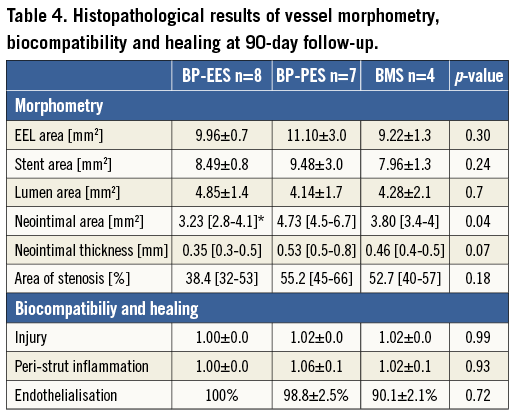
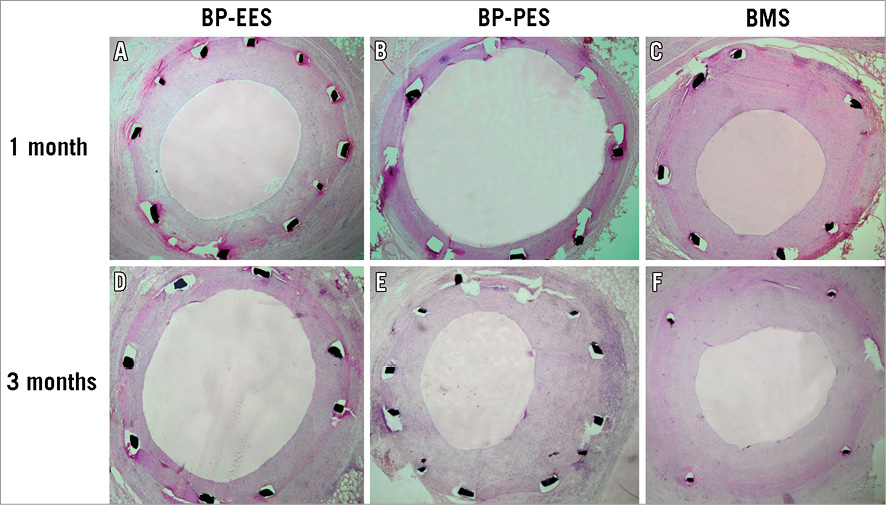
Figure 2. Representative histopathological images of BP-EES, BP-PES and BMS, cross-sections at one (A-C) and three-month (D-F) follow-up stained with haematoxylin and eosin. BMS: bare metal stent; BP: biodegradable polymer; EES: everolimus-eluting stent; PES: paclitaxel-eluting stent
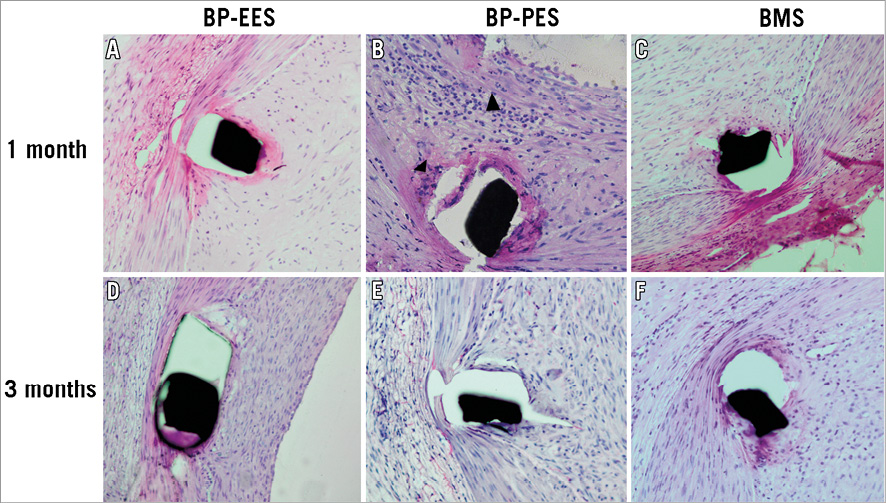
Figure 3. Representative high power images of peri-strut magnifications (20x): BP-EES, BP-PES and BMS cross-sections at one (A-C) and three-month (D-F) follow-up stained with haematoxylin and eosin. Note inflammatory cell response in BP-PES at one month (black arrowheads). BMS: bare metal stent; BP: biodegradable polymer; EES: everolimus-eluting stent; PES: paclitaxel-eluting stent
TEMPORAL VASCULAR HEALING RESPONSE
The analysis of results between 28 and 90 days (Figure 4, Figure 5) showed that in BP-EES neointimal hyperplasia stabilised after one month. On the other hand, a significant increase in neointimal hyperplasia over time occurred in BP-PES, with an almost threefold increase in late loss (p=0.04) and fourfold increase in neointimal thickness (p<0.01). In the BMS group, there was a trend (26%) toward an increase in neointimal thickness (0.31±0.1 vs. 0.44±0.1 mm; p=0.09), whereas no differences were noted in late lumen loss. At 28 and 90 days, the endothelialisation process was complete and comparable in EES (p=1.0), whereas it was moderate at 28 days, with a significant improvement over time in PES (p<0.01). Similarly, inflammation was low and comparable in EES (p=1.0), whereas it was mild and low in PES at 28 and 90 days, respectively (p<0.01).
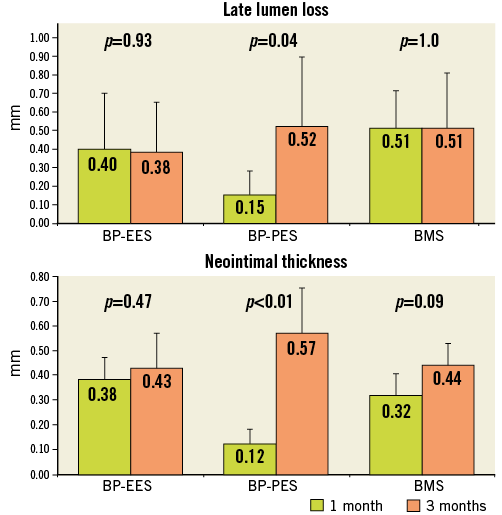
Figure 4. Temporal vascular healing response. Neointimal hyperplasia at one and three-month follow-up among groups expressed as late lumen loss in angiography and neointimal thickness in histological evaluation. BMS: bare metal stent; BP: biodegradable polymer; EES: everolimus-eluting stent; PES: paclitaxel-eluting stent
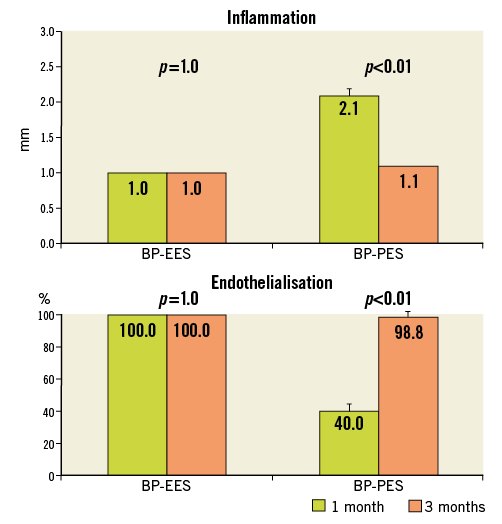
Figure 5. Temporal vascular healing response. Inflammation and endothelialisation at one and three-month follow-up. BMS: bare metal stent; BP: biodegradable polymer; EES: everolimus-eluting stent; PES: paclitaxel-eluting stent
Discussion
In our study, we evaluated the independent role of two different antiproliferative agents released from identical biodegradable polymer-stent matrices on vascular neointimal response, biocompatibility and safety in a well validated porcine coronary injury model13. At one month, paclitaxel-eluting stents showed the highest degree of inhibition of neointimal hyperplasia, as evidenced by angiography and histology, at a cost of delayed healing. Although everolimus-eluting stents did not achieve a similar inhibitory effect at 28 days, they seemed to have better biocompatibility and healing. Otherwise, at long-term follow-up, measures of neointimal hyperplasia and vessel stenosis were lowest in BP-EES when compared to BP-PES and BMS, showing numerically smaller values than at one month. In contrast, there was a 30% increase in neointimal formation in BMS and an almost fourfold increase in paclitaxel stents. Interestingly, a discrepancy between late loss and %DS in BP-PES was noticed at 28-day follow-up. This might have been caused by the edge effect related to excessive neointimal hyperplasia or impaired relaxation in the reference segments. However, this must be interpreted cautiously due to the low resolution of angiography, especially in the low grades of stenosis caused by DES14.
To the best of our knowledge, this is the first study which has discriminated the independent effect of paclitaxel and everolimus on vascular response eluted from identical stent-polymer matrices. Additionally, drug concentrations encapsulated in polymer were equivalent. Similar attempts to distinguish the autonomous roles of biodegradable versus durable polymer in similar stent-drug matrices were reported in the preclinical and clinical setting, showing promising results for polymer biodegradation3,15. In our study, focusing on the role of a drug, a very common neointimal catch-up effect of paclitaxel-eluting stents was observed. However, in studies that have described this phenomenon, it is unclear whether polymer or the drug itself contributed to this effect16-18. The findings from this experiment allow us to disavow the influence of polymer and stent platform in this effect, emphasising the role of the drug itself and its pharmacokinetics. This seems to be supported by good clinical and angiographic results after paclitaxel-coated balloon angioplasty of restenosis19,20, achieved after single drug delivery and with very low paclitaxel concentrations in the vessel wall at long-term follow-up21. Therefore, maintaining high levels of paclitaxel constantly delivered from a polymer-stent matrix at longer follow-up may cause paradoxical effects and contribute to neointimal catch-up in the longer term. This has been shown in one of our previous studies in which local delivery of high-dose paclitaxel via a microporous catheter resulted in paradoxical profound neointimal formation22.
In the everolimus-eluting stent group, high biocompatibility and complete endothelialisation were already shown at one month, confirming a favourable safety profile. Although the efficacy in terms of neointimal inhibition was not shown at one month, it seemed to increase over time, showing lowest neointimal hyperplasia at three months among groups with a 30% decrease of neointimal formation when compared to BMS, and an almost fourfold decrease when compared to BP-PES. The lack of efficacy of everolimus-eluting stents at one month can be explained by the use of a healthy domestic swine model, which is well established for evaluation of safety and biocompatibility; however, assessment of efficacy remains limited13,23. This was confirmed in a similar preclinical evaluation of a well-established everolimus-eluting stent (XIENCE; Abbott Vascular, Redwood City, CA, USA) where lack of neointimal inhibition at short-term follow-up was also reported24. Interestingly, efficacy of EES was displayed in the novel model of familial hypercholesterolaemic swine.
A preferable biocompatibility profile of tested everolimus-eluting stents with very low peri-strut inflammation at each study point is supported by previously published reports suggesting that stent-based delivery of everolimus selectively decreases inflammation by clearing macrophages by autophagy, an mTOR inhibition-dependent mechanism inducing cell death25. Similarly, the endothelialisation process in PES and EES at 28 days confirms the observations arising from cell culture studies4,9 where paclitaxel significantly inhibited the migration of endothelial cells in contrast to sirolimus.
A prospective, clinical randomised trial evaluating coronary revascularisation with identical drug-eluting stents was reported recently26. At nine-month angiographic follow-up, the BP-EES were comparable to BP-PES with regard to late lumen loss (0.31 vs. 0.3 mm, p=0.94), which seems to resemble the angiographic outcomes in current experimental study.
Limitations
The limitations of this study include the nature of an experimental preclinical model as a human clinical surrogate and the utilisation of healthy domestic swine, without underlying disease. Furthermore, no intravascular imaging was utilised to prevent injury to endothelium caused by mechanical pullback in order to provide unbiased histopathological results. Histopathological evaluation was performed with H&E staining only.
Conclusions
In summary, temporal differences in vascular response were seen by the delivery of different antiproliferative agents from identical polymer-stent matrices. Basing on the current findings, we conclude that paclitaxel seems to induce a higher degree of inflammation in the short term leading to higher degrees of neointimal hyperplasia in the long term. On the other hand, the delivery of everolimus was associated with preferable healing and biocompatibility at short-term follow-up, which resulted in improvement in neointimal inhibition at long-term follow-up.
| Impact on daily practice Based on this experimental study, the local delivery of everolimus after coronary revascularisation may offer a similar outcome to paclitaxel with regard to restenosis inhibition. However, evidence of complete early healing was observed only after delivery of everolimus, which may translate into lower thrombotic events in the clinical setting and development of novel local delivery technologies of this drug. |
Funding
Balton Ltd (Warsaw, Poland) provided financial support and materials for this study.
Conflict of interest statement
K. Milewski receives consulting fees from Balton. B. Gwiazdowska-Nowotka is a full-time employee of Balton. The other authors have no conflicts of interest to declare.

