Abstract
Aims: The vascular effects of drug- eluting balloon (DEB) deployment in the absence of coronary stents have not been characterised. This study evaluated potential vascular effects of paclitaxel-coated angioplasty balloons using different excipients in the absence of additional stents.
Methods and results: A total 45 porcine arteries were treated with paclitaxel-coated DEBs using four different excipients (all 3.0 µg/mm2): A) iopromide (n=9), B) ATEC excipient (n=8), C) BTHC excipient (n=10), D) lecithine excipient (n=10). Uncoated bare angioplasty balloons served as controls (n=8). Histology, histomorphometry, and quantitative angiography analysis were performed 28 days following intervention. Tissue concentrations of paclitaxel were measured in selected animals using BTHC excipient (n=39 arteries) and reached maximum concentrations of 165 ng/mg 30 min after delivery in coronary target tissue. There were no differences in efficacy endpoints using histomorphology or quantitative angiography between groups. In contrast, however, treatment with DEBs using BTHC excipient or iopromide was associated with increased fibrin deposition and inflammation indicating delayed vascular healing. DEBs using lecithin excipient or uncoated angioplasty balloons did not induce any comparable vascular effects.
Conclusions: Effective excipients are necessary to accomplish successful balloon facilitated paclitaxel delivery, which is associated with delayed vascular healing as a sign of successful drug transfer. The potential of DEBs to diminish restenosis following angioplasty may be insufficient in the absence of additional stents.
Introduction
The introduction of drug-eluting stents (DES) has markedly improved percutaneous treatment of coronary artery disease, however, current DES inherently lack sufficient efficacy in challenging lesion subsets1-3. Drug-eluting balloons (DEBs) have recently been identified as alternative treatment in patients with recurrent in-stent restenosis (ISR), femoropopliteal atherosclerotic disease as well as de novo lesions in small vessels4-6.
To date, clinical evidence for the efficacy of DEBs has been established in patients with in-stent restenosis and femoropopliteal atherosclerotic disease7-10. The success of DEBs in treating ISR or femoropopliteal artery disease is likely a result of effective drug retention within the neointimal tissue and the advanced peripheral atherosclerotic plaque, respectively. Due to the limited efficacy of DES in small vessels11, DEBs may represent a promising approach in this context. However, the precise vascular effects of paclitaxel-based angioplasty in the absence of supporting stents have not been characterised. Moreover, previous studies in this regard demonstrated the importance of appropriate choice and application of drug and excipient12. Paclitaxel is a highly effective anti-proliferative agent that has successfully been implemented in clinically effective paclitaxel-eluting stents13,14. However, due to its insufficient solubility in water and crystalline physical nature, specific solvents are necessary for an angioplasty-based application of this drug. While previously established organic solvents like ethoxylated castor oil represent effective candidates as solvents for paclitaxel, their bio-incompatible nature remains an insuperable obstacle for their practical application as excipients utilised in DEB technology.
The aim of this study was, therefore, to evaluate the capability of four different excipients to facilitate successful tissue transfer of paclitaxel in the absence of stents in a porcine model of angioplasty and to characterise the vascular effects of angioplasty-based paclitaxel transfer against the different additives used.
Methods
Care and use of animals in the present studies were in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) as well as German national guidelines.
Balloon coating
All tested DEBs consisted of identical conventional semi-compliant angioplasty balloon catheters (Pantera™, 3.5×20 mm; Biotronik, Erlangen, Germany) coated with paclitaxel (all 3.0 µg/mm2) using four different excipients: 1) iopromide coating as used on the commerial SeQuent® Please catheter (iopromide group)6 (B.Braun Melsungen AG, Berlin, Germany), 2) ATEC (acetyltriethyl citrate) excipient (ATEC group) 3) BTHC (n-butyryl-tri-n-hexylcitrate) excipient (BTHC group), 4) lecithin excipient (lecithin group). The same but uncoated angioplasty balloon catheter served as a control (control group).
Animal model and balloon deployment
A total of 34 male and female non-atherosclerotic healthy Landrace Yorkshire domestic pigs were included in the study. Nineteen animals were used to evaluate the vascular effects of DEBs utilising different excipients and uncoated control balloons following angioplasty. Animals were treated with aspirin 325 mg and clopidogrel bisulfate 75 mg daily. After intramuscular injection of ketamine (20 mg/kg), xylazine (5 mg/kg), and atropine (0.05 mg/kg), anaesthesia was initiated by intravenous injection of propofol (2 mg/kg). Orotracheal intubation was performed and anaesthesia was maintained using isoflurane 1.5-3.0% (2 L/min). An intravenous bolus dose of heparin was administered at a dose of ~300 IU/kg.
Coronary angiography was performed using standard contrast medium in two orthogonal views (LAO and RAO respectively) after intracoronary nitroglycerine (200 μg) administration. Using fluoroscopic control a vessel section of adequate dimensions was chosen.
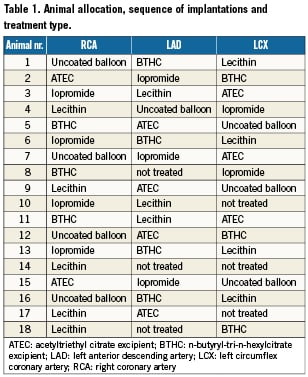
All balloon inflations were performed with an inflation pressure of 6 atmospheres and 60 seconds inflation time. The three major coronary arteries of the animals were randomly assigned to one of the four treatment groups according to a pre-specified allocation table (Table 1). Angiographic evaluation (QCA) was performed before, during and after treatment. Twenty eight days after intervention, each treated vessel underwent follow up angiography and quantitative coronary angiography (QCA) analysis. After final angiographic examination, animals were euthanised using pentobarbital sodium 390 mg/ml, 1% propylene glycol, 29% ethyl alcohol, 2% benzyl alcohol.
Quantitative coronary angiography (QCA)
Analysis of coronary angiography images was performed using representative single digital images selected from the digital video sequences. The following standard parameters were assessed: 1) mean lumen diameter of the treated vessel segment, 2) MLD (minimal lumen diameter), 3) percent diameter stenosis, 4) balloon to artery ratio, 5) late lumen loss (MLD post balloon intervention - MLD at follow-up) and 6) late lumen loss index ([MLD post balloon intervention - MLD at follow-up]/ MLD post balloon intervention).
Histopathology and histomorphometry
The complete heart and the vessels were excised immediately and submitted for histopathologic evaluation. Treated coronary segments underwent gross pathological evaluation for epicardial haemorrhage, aneurysms, thrombus formation or dissections.
Vascular injury was graded according to the degree of local tissue damage, and the extent relative to the vessel circumference. A composite score was derived as shown below (Table 2). The degree of tissue damage was defined as the following: 1 = circumscribed rupture of the IEL, without involvement of the media; 2 = laceration or rupture of the media involving the IEL; 3 = laceration or rupture of the adventitia involving both the IEL and media. The extent of vessel damage was classified as involving less than one quarter of the vessel circumference, between one quarter and half or more than half of the vessel circumference.
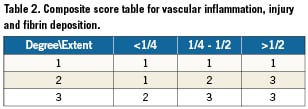
In a similar fashion, vascular inflammation was graded according to the degree of inflammatory cell infiltrates within the intimal tissue as the following: 1 = modest infiltration of neutrophils, monocytes and macrophages; 2 = moderate infiltration of neutrophils, monocytes and macrophages; 3 = severe infiltration of neutrophils, monocytes and macrophages. Along similar lines, composite scores were derived for inflammation and intimal fibrin deposition, based on extent of changes and relative vessel circumference involved (Table 2). The histologic section of the artery was also examined for endothelialisation using a semi quantitative score (1 = <25%, 2 = 25 – 75%, 3 = >75% endothelial cell coverage).
Paclitaxel concentration measurements
Tissue concentration analysis was performed in selected animals (n=15) treated with DEBs utilising BTHC excipient. Paclitaxel concentrations were measured in blood, target vessels as well as in downstream tissues following 60 seconds balloon inflation at 6 atmospheres. Blood was collected at 5±1 min, 30±5 min, 120±15 min, 8±0.5 hours, 24±2 hours, 72±6 hours, and 7±1 days in order to evaluate systemic drug pharmacokinetics. After sacrifice, myocardium of interest and the treated vessels were harvested, snap frozen, homogenised and submitted for concentration analysis following appropriate purification steps. The concentrations of paclitaxel were measured in blood and tissue using a validated liquid chromatography- tandem mass spectrometry (LC-MS/MS) assay.
In brief, a validated method for the quantification of paclitaxel has been utilised. Liquid chromatography paired with mass spectrometry is often regarded as the gold standard for analysis since analytes are separated by chromatography, then selectively identified and measured by mass spectrometry. In the case of this assay, a triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) was utilised, which allowed for mass selection in the first quadrupole followed by fragmentation and fragment mass selection (column: Agilent, Zorbax, XDB-C18, 150 mm×5 μM, mobile phase: Acetonitrile, 0.01% formic acid in water). Because of the sensitivity of the assay, which can detect low picogram concentrations of paclitaxel, several samples in the study needed to be diluted into the linear range of response for the assay. Dilution integrity was demonstrated in the method validation.
Statistics
Quantitative coronary arteriography. QCA data are represented as mean±standard deviation. Comparisons between the treatment groups were performed using Wilcoxon rank sum (SAS). A value of < 0.05 was considered statistically significant.
Histomorphometric analysis. Continuous morphometric data are represented as mean±standard deviation. A Wilcoxon Rank Sum test was used to test each of the paired comparisons between the balloon groups and stent groups. A value of <0.05 was considered statistically significant.
Results
Of the thirty-four animals included, thirty-three animals survived until the scheduled follow-up time point of 28 days. One animal died during recovery of the treatment procedure. Pathological evaluation of the heart showed the LCX with extensive medial dissection at the balloon-treated segment while acute haemorrhage streaks were noted at the anterior and antero-septal wall of the left ventricle.
A total of 19 animals (57 arteries) were allocated for the investigation of vascular effects following DEB or control angioplasty, while 15 animals (45 arteries) were used for tissue concentration analysis of paclitaxel.
Of the 57 arteries allocated to DEB or control angioplasty, nine vessels were excluded due to unfavourable arterial anatomy (i.e., small vessel dimensions). Another three vessels were lost due to the acute death described above, which left a total of 45 vessel treated by DEBs (n=9 for the iopromide group, n=8 for the ATEC exipient group, n=10 for the BTHC excipient group and n=10 for the lecithine excipient group) or control angioplasty (n=8).
Of the 45 arteries allocated for tissue concentration analysis, a total of six arteries were used for each consecutive time point and separated equally among the LAD, LCX and RCA (n=2 each), with the exception of the eight hours cohort (n=3 for the LAD, n=2 for the LCX, n=3 for the RCA), and the three days cohort (n=2 for the LAD, n=2 for the LCX and n=3 for the RCA).
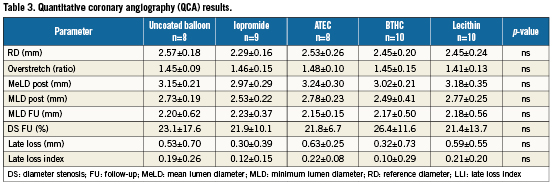
Quantitative coronary angiography
Quantitative coronary angiography revealed comparable baseline characteristics between groups. Furthermore, the degree of balloon/ vessel oversize was similar among all study groups (Table 3). At 28-day follow-up, minimum lumen diameter (MLD) and mean diameter stenosis (DS) were not significantly different between groups. Late lumen loss (LLL) and the late loss index (LLI), however, were numerically lower in the iopromide and BTHC groups compared to control (Table 3).
Morphometry and histopathology
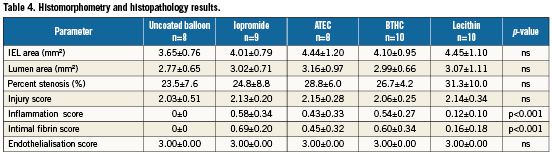
External gross examination of the hearts did not show any abnormalities along the course of the coronary arteries other than mild expansion of the artery at the balloon treatment site. Pathological examination revealed absence of vascular haemorrhage, aneurysms, dissections or thrombus formation. There was no significant difference in the morphometric assessment among the treatment groups (Table 4).
Vascular signs of successful paclitaxel transfer
Regarding vascular injury, there were no significant differences among groups (Table 4). Successful paclitaxel transfer was determined after complete histopathologic assessment and analysis of tissue concentrations within the treated segments. Successful paclitaxel transfer was associated with increased inflammation and fibrin deposition. In line with this, the use of DEBs utilising iopromide or BTHC excipients was associated with significantly higher inflammation and fibrin scores compared to uncoated control balloons (Table 4).
Furthermore, in comparison to deployment with uncoated control balloons, where intimal fissures and dissections were almost healed, the use of DEBs with iopromide or BTHC excipient resulted in delayed intimal healing (Figure 2). Against this, deployment of DEBs utilising lecithin as excipient resulted in inflammation scores similar to the uncoated control balloon while fibrin deposition was absent. Deployment of DEBs utilising ATEC as excipient resulted in moderately increased inflammation and fibrin scores, representing an intermediate potential in paclitaxel transfer.
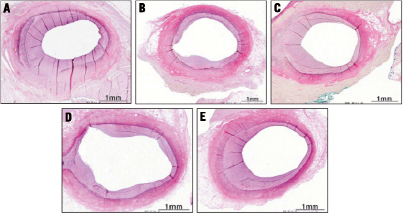
Figure 1. Representative VVG stained coronary sections following deployment of uncoated control balloons (A), DEBs utilising iopromide (B), ATEC (C), BTHC (D) or lecithin (E) as excipients. Note, there is reduction in restenosis observed with the BTHC (D) and the iopromide (B) groups.
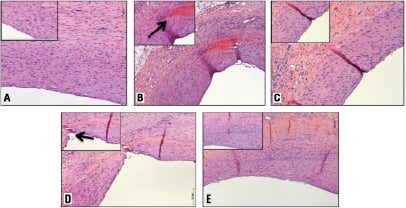
Figure 2. Representative H&E stained coronary sections following deployment of uncoated control balloons (A), DEBs utilising iopromide (B), ATEC (C), BTHC (D) or lecithin (E) as excipients. Note, the BTHC (D) and the iopromide (B) groups are accompanied by increased inflammation and fibrin depositions within unhealed neointimal fissures (inlets, black arrows).
Paclitaxel concentration analysis
Overall pharmacokinetic analysis showed that after balloon delivery in vivo, paclitaxel is successfully released from the test device into the circulation and target tissues. After use of the DEB using BTHC excipient, the pharmacokinetic profile of paclitaxel in blood showed a maximum concentration of 174 ng/mL five minutes after exposure to the DEB (Figure 3). The average terminal half-life was 46 hours. Paclitaxel reached maximum concentrations of 165 ng/mg 30 min after delivery in coronary target tissue with an exponential decline over seven days. The maximum concentrations were measured in the middle section of the treated segment (Figure 4).
Paclitaxel tissue distribution showed a concentration gradient, with maximum concentrations observed in the myocardial tissue adjacent to the treated coronary segment, and a continuous decline towards the remote myocardial segments.
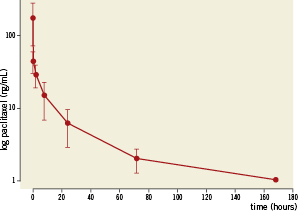
Figure 3. Log transformed paclitaxel concentration measured in blood along different time points.
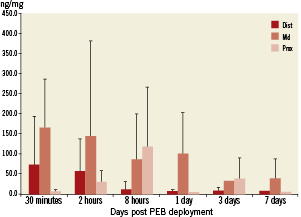
Figure 4. Paclitaxel coronary tissue concentration measure by liquid chromatography- tandem mass spectrometry (LC-MS/MS) analysis. Note, peak levels of paclitaxel were detected within the middle sections of the coronary segments 30 minutes after balloon deployment. There is persistence of paclitaxel within the coronary tissue up to seven days following balloon deployment.
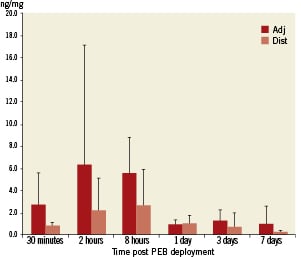
Figure 5. Paclitaxel myocardial tissue concentration measure by liquid chromatography- tandem mass spectrometry (LC-MS/MS) analysis in adjacent (dark bars) and distal myocardial segments (light bars). Note, peak levels of paclitaxel were detected within the myocardial sections adjacent to the treated coronary segments two hours after balloon deployment.
Discussion
The goal of the current study was to characterise the principle vascular reactions after successful angioplasty-based paclitaxel transfer. In this regard the main findings of this study were:
(i) Deployment of a DEB using BTHC as excipient resulted in vascular effects similar to those observed in commercial DEBs (using iopromide as excipient) that had previously been proven effective in man7,10,15.
(ii) Deployment of DEBs using lecithin as excipient resulted in vascular effects similar to uncoated control balloons, which likely reflects the failure of effective paclitaxel transfer, while DEBs using ATEC as excipient potentially displayed an intermediate drug transfer potential.
(iii) The principle vascular effects observed following DEB deployment strongly depend on the extent of successful drug transfer into the vessel wall. While balloon angioplasty in combination with low-efficacy drug transfer resulted in moderate elastic recoil and injury-induced neointimal growth in the presence of complete neointimal healing, the attenuation of this phenomenon by means of reduced neointimal growth, increased vascular inflammation and fibrin deposition could be documented as sign of effective drug transfer.
(iv) The magnitude of restenosis inhibition utilising angioplasty-based paclitaxel delivery is considerably lower compared to the stent-supported application of DEBs in the porcine coronary model. It has to be accepted, therefore, that the animal model of plain angioplasty is probably not suitable to investigate changes in neointimal proliferation after use of current DEBs.
The importance of excipients
One of the most important findings of the early work performed by Scheller et al was the observation that paclitaxel coating of plain balloon catheters in the absence of excipients seemed to be insufficient to achieve adequate inhibition of neointimal proliferation as compared to catheters coated with paclitaxel in combination with the hydrophilic X-ray contrast medium iopromide16. In this context, a number of different coating strategies failed to show sufficient efficacy in neointimal inhibition12. Our findings strongly support the pivotal role of excipients in the coating technology of DEBs.
As with the well characterised coating of the SeQuent® Please DEB, a porous surface is applied to the Pantera™ balloon angioplasty catheter, where a large contact area is created between the lipophilic drug molecules and the vessel wall leading to uniform and complete release of the drug after the first balloon expansion. Similarly, absorption properties of paclitaxel are enhanced by the addition of BTHC (10:1 ratio of paclitaxel: BTHC), which keeps paclitaxel micro-crystalline in structure. These attributes result in a high bioavailability of paclitaxel at the target site, a pre-requisite for rapid drug absorption into the vessel wall. Accompanying pharmacokinetic studies of DEBs using BTHC as excipient demonstrated that target tissue concentrations are well within a therapeutic range known from cell culture experience with paclitaxel, with equipotent inhibition of coronary artery smooth muscle and endothelial cells (effective from 56 nmol/l to 50 mmol/l)17 as well as other preclinical studies using effective DEBs18.
On the other hand, deployment of the same DEB catheter using lecithin as excipient did not result in vascular effects that were observed in clinically proven iopromide-coated DEBs, which importantly underscores the relevance of the excipient in the application of DEBs.
Along similar lines, the use of ATEC as excipient resulted in less effective paclitaxel transfer to the vessel wall compared to the use of BTHC. Several potential explanations may be offered in this regard. As the solubility of ATEC in water is considerably higher compared to BTHC, its relative hydrophilicity may prevent paclitaxel from dissolving, and hence may promote rapid wash-out effects of the drug during deployment of the DEB. Further, utilising ATEC as excipient may result in incompatible coating layers that may result in early loss of the drug during transition to the lesions.
Taken together, these data clearly indicate that successful application of DEBs requires the use of an effective coating, facilitating vascular delivery of antiproliferative drugs.
Vascular signs of effective paclitaxel transfer
The vascular effects of DEB-based paclitaxel transfer also resemble well-studied biological effects of paclitaxel-eluting stents (PES). Paclitaxel as a cytotoxic drug suppresses smooth muscle –as well as endothelial cell proliferation and migration in adequate concentrations. After transfer into the vessel wall, the resulting reduction of neointimal growth is accompanied by persistent fibrin deposition, macrophage infiltration and an overall decrease in smooth muscle cells in rabbit iliac and porcine coronary arteries19. These biological effects induced by clinically effective paclitaxel-eluting stents share important similarities to the vascular reactions observed after DEB treatment in our study. Here, we also documented a clear signal regarding increased inflammatory responses and fibrin deposition in those coronary arteries treated by iopromide or BTHC facilitated paclitaxel transfer.
Prolonged fibrin depositions as well as increased inflammatory reactions have both been reported as markers of delayed arterial healing after DES implantation20. More specifically, PES have been associated with greater fibrin deposition compared to sirolimus eluting stents, which was reported an important hallmark of drug efficacy within the vascular tissue21. Therefore, the existence of increased fibrin deposition after DEB treatment likely mirrors effective paclitaxel transfer following DEB deployment.
In contrast to the findings with PES in rabbit iliac arteries19, endothelialisation was complete among the treatment groups in the current study. This may be a result of the 28 day time-point chosen for final examination, which may not be appropriate for assessing endothelialisation in the porcine model. Previous studies suggested that, unlike in humans, endothelialisation may be complete by 14 days using a number of DES19. Furthermore, complete endothelialisation likely occurs much earlier after balloon-facilitated drug transfer as compared to stent (DES) mediated drug release.
The clinical implications of increased fibrin deposition and inflammation in our study are as yet unclear. In comparison to PES, however, adverse effects consequent on durable polymer residue –a significant contributor to inflammation and delayed healing– is not an issue with DEB therapy. The time course of delayed healing in DEB-treated, as compared to DES-treated coronary segments may therefore be considerably shorter.
DEB deployment in an animal model of plain angioplasty
Another unique aspect of this preclinical study refers to the animal model chosen. Most previous preclinical studies evaluating DEBs in porcine coronaries used stents as scaffolding device (simultaneous or sequential implantation)6,7,8,12,16. As a result, DEBs primarily reduced restenosis by suppressing in-stent neointimal growth as compared to uncoated balloons. Furthermore, when using stents precrimped on DEBs, reduced periprocedural drug washout in transition to the target lesion may significantly modify drug release characteristics and impact on the magnitude of neointimal growth inhibition in preclinical studies as well as in man.
In the current study, we aimed to determine the vascular effects of successful paclitaxel transfer in a porcine coronary angioplasty model in the absence of stents. As the biological processes underlying the emergence of restenosis following balloon angioplasty differ substantially from those occurring after stent implantation, pharmacological interventions exclusively aiming at reductions of neointimal growth may insufficiently suppress restenosis after pure angioplasty, at least in the porcine coronary model. The impact of this findings deserves further investigation in dedicated clinical studies. This important notion is impressively resembled in our study, where efficacy endpoints did not differ significantly among groups despite successful paclitaxel transfer. As a result, it has to be accepted that the animal model of plain angioplasty is not suitable to investigate changes in neointimal proliferation after use of current DEB systems. Furthermore, it might be speculated that DEBs are not capable to reduce coronary restenosis at all when used without stent support. In turn, previous preclinical studies evaluating DEBs in combination with bare- metal stents are less suited for detecting the vascular effects of balloon-facilitated paclitaxel transfer in the absence of supporting scaffolds.
Limitations
This study was carried out in juvenile domestic pigs following overstretch injury.
The non-diseased coronary arteries of juvenile pigs are likely to react differently to balloon-mediated injury when compared to human atherosclerotic coronaries. Moreover, paclitaxel tissue uptake may differ significantly in atherosclerotic lesions due to the greater accumulation of the drug inside the atherosclerotic plaque.
Nevertheless, non-diseased animal models remain an important tool to study the vascular effects of novel transcatheter devices due to the inherently lower variability in injury-mediated vascular reactions.
Commercially available SeQent® Please balloons were not used. Instead, a similar coating was used on identical Pantera™ balloons. The results may, therefore, potentially differ from commercially available Sequent® Please balloons.
Finally, pharmacokinetic data in blood and target tissues have been limited to the BTHC excipient.
Acknowledgements
This work was funded by Biotronik SE & Co. KG. The authors gratefully acknowledge Ron Waksman, MD and David Hellinga, MSc from the Cardiovascular Research Institute, Washington DC, USA for their excellent support in conducting the animal experiments for the safety and efficacy study. We thank Capucine Lapointe-Corriveau, MSc and Louis-Georges Guy, PhD from AccelLAB Inc., Montreal, Canada for their excellent support in conducting the animal experiments for the pharmacokinetic study. We thank Uwe Christians, MD, PhD and Eurofins Medinet for their excellent analytical work.
Conflict of interest statement
P. Radke and M. Joner have received speakers’ and consultcy fess from Biotronik. S. Hartwig, G. Bayer and E. Wittchow are empolyees of Biotronik. The other authors have no conflict of interest to declare.

