Introduction
Percutaneous transluminal treatment of vascular disease represents one of the most innovative fields of modern medical research. In coronary interventions the most important improvement of angioplasty has been achieved with the introduction of stents. Stenting overcomes recoil and dissections, but not restenosis due to neointimal proliferation. Local intravascular drug delivery by drug-eluting stents seemed to cross one of the last frontiers in interventional procedures, namely restenosis. However, stents cannot be implanted at all sites where neointimal proliferation reduces the long-term benefit of angioplasty. Furthermore, drug-eluting stents were not effective in the treatment of peripheral vascular disease. In the coronary field, delayed and incomplete endothelialisation with the risk of late stent thrombosis may limit the widespread use of this technology.
Drug-coated balloons may overcome some limitations of drug-eluting stents and represent an additional platform in the toolbox of interventional cardiology and angiology. This article summarises the history, conception, preclinical and early clinical findings with the first drug-coated balloon: “from bench to Paccocath”.
History of the drug-coated balloon project
Stimulated by early scientific reports of local drug applications, Ulrich Speck and Bruno Scheller were looking for innovative methods to influence the process of neointimal proliferation apart from stent-based drug delivery. One of their first key experiments was the addition of an antiproliferative drug to a contrast agent.1 The contrast agent served as a carrier for local intravascular drug delivery. It improved the solubility of the drug far beyond the concentrations applied in previous investigations. In the porcine coronary model, the intracoronary bolus administration of a taxane-iopromide formulation led to a significant reduction of neointimal formation after experimental coronary stent implantation, despite the short application time.2,3
In the next step, the working group looked for a more lesion- than vessel-specific method of intravascular drug delivery. The idea of adrug-coated balloon providing a similarly short lasting application was further pursued and first in vivo experiments were envisaged. Being aware of the drug-eluting stent euphoria, animal trials with different paclitaxel-coated balloon prototypes started in 2002.
Preclinical evaluation of the drug-coated balloon
There were extensive in vitro and in vivo experiments investigating the coating method, adherence and release of the drug. The initial studies in the porcine coronary stent model addressing neointimal proliferation found a specific matrix coating (Paccocath) with paclitaxel admixed to a small amount of the hydrophilic X-ray contrast medium iopromide (Ultravist) to be effective. Paccocath balloons are standard angioplasty balloons coated with a paclitaxel dose of 3 µg/mm2 balloon surface. Iopromide used as a matrix enhances the dissolution and release of the drug and also assists in its adherence to the vessel wall.
First proof of concept
The aim of the first animal study was to test the efficacy of paclitaxel-coated balloons in respect to drug transfer and reduction of neointimal formation in the porcine coronary model. In the first trial, different coatings and dosages were studied.4
Conventional angioplasty balloon catheters (length 20 mm, diameter 3.0 and 3.5 mm) were coated with paclitaxel using two different procedures: procedure EEE used ethyl acetate as solvent and resulted in about 2 µg paclitaxel/mm2 balloon surface; procedure Ac used acetone as solvent and was done with two concentrations of paclitaxel (low dose resulting in 1.3 µg/mm2, AcL; and regular dose resulting in 2.5 µg paclitaxel/mm2, AcR).
To determine the uptake of paclitaxel into the vessel wall, domestic pigs received paclitaxel coated and non-coated balloon catheters with or without pre-mounted stents. Coated balloon catheters with and without stents were advanced into the coronary arteries, where they were left un-inflated in the blood stream for approximately five minutes and then withdrawn. The paclitaxel content of the balloons was determined by HPLC after extraction and compared to the paclitaxel content of unused catheters of the same batch. In the same animals three different treatments were performed in randomised order:
– implantation of bare stent pre-mounted on paclitaxel-coated balloon catheter (Ac-coating regular dose).
– angioplasty with paclitaxel-coated balloon catheter (Ac-coating regular dose) without stent implantation; to label the site of dilatation a short stent was implanted distal to it before PTCA was performed.
– bare stent implantation with a non-coated balloon. Thereafter, post-dilatation of the stented segment with a paclitaxel-coated balloon catheter (Ac-coating regular dose) was performed.
After 40-60 minutes, treated coronary artery segments from the LAD, Cx and RCA were dissected. Paclitaxel was extracted from homogenised tissue samples with dimethyl sulfoxide and from plasma samples with ethyl acetate using docetaxel as an internal standard. Samples were analysed by HPLC. Introduction of coated balloon catheters without stents through the delivery sheath to the coronary arteries and retraction without inflation resulted in a 6% loss of the active ingredient (paclitaxel content 94±10% of initial dose; n=4). Folded balloons with mounted stents showed no significant loss of paclitaxel during five minutes of floating (paclitaxel content 102±6% of initial dose; n=3). After coronary artery dilatation most of the drug was released during balloon inflation resulting in the transfer of effective doses to the vessel wall.
In the restenosis part of the study, stents were implanted with an oversize ratio of about 1.2. According to the randomisation list, the pigs received a total of 40 identical stents into LAD and Cx arteries. The stents were pre-mounted on identical balloon catheters which were uncoated or coated in three different ways:
– control: bare stents crimped on conventional uncoated PTCA balloon catheters; n=13
– EEER: bare stents crimped on paclitaxel coated PTCA balloon catheters using the EEE coating technique with regular dose; n=10
– AcL: bare stents crimped on paclitaxel coated PTCA balloon catheters using the Ac coating technique with low dose; n=10
– AcR: bare stents crimped on paclitaxel coated PTCA balloon catheters using the Ac coating technique with regular dose; n=11
After five weeks, the animals underwent control angiography, target segments were dissected from the heart, and samples for histology obtained. There were no acute or subacute thrombotic complications and no significant adverse events in terms of ECG parameters, blood pressure, or contractility during or after the coronary interventions. After inflation, stent deposition and withdrawal through the guiding catheter, the paclitaxel content of the balloons was measured. The postprocedural content was 13±2% of dose on the EEER balloons (n=10), 10±2% on the AcL balloons (n=10) and 7±2% on the AcR balloons (n=12), respectively.
The Ac coating procedure resulted in a marked, dose-dependent and statistically significant reduction of late lumen loss and an equally impressive, statistically significant increase in minimal lumen diameter compared to controls (Figure 1). Quantitative coronary angiography revealed no edge effects. Histomorphometry showed a statistically significant increase in lumen diameter and lumen area, and a corresponding decrease in maximal neointimal thickness and neointimal area in the vessels treated with AcL- and AcR-coated balloons. The AcL paclitaxel coating led to a reduction of the neointimal area by 42%, the AcR coating by 72%, respectively. Despite the marked reduction of neointimal proliferation, endothelialisation of stent struts was present in all samples. There was no evidence of a significant inflammatory response in the neighbourhood of the stent struts.
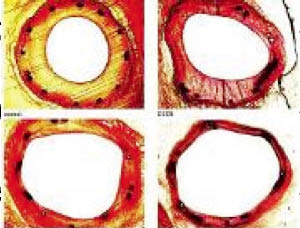
Figure 1. Results of histomorphometry of stented porcine coronary arteries after 35 days.
Dose finding
One coating method (Ac) in the above study was efficient in reducing neointimal formation in the porcine coronary model.4 Nevertheless, little was known at this point in time about the most suitable dosages of what we now call the Paccocath coating.
Therefore, the aim of an additional animal study was to evaluate the safety and efficacy of different paclitaxel dosages on neointimal proliferation in the porcine coronary overstretch model.5 Sixty-four stainless steel stents (diameters, 3.0 and 3.5 mm; length, 19 mm) were implanted in the LAD and Cx coronary arteries of 32 domestic pigs. Both conventional uncoated and paclitaxel-coated DCB balloon catheters were used. The animals were randomised to three different treatments, with coatings of 2, 3, and 6 µg paclitaxel/mm2 balloon surface. After 28 days, quantitative angiography and histomorphometry of the stented arteries was performed.
Paclitaxel balloon coating led to a marked reduction of neointimal thickness independent of the applied dose. Quantitative coronary angiography revealed a highly significant (p<0.001) reduction of late lumen loss for the 3 and 6 µg/mm2 treatment. However, a lower dose of 2 µg/mm2 led to a less pronounced reduction of late lumen loss (Figure 2). Despite the marked reduction of neointimal proliferation, endothelialisation of stent struts was present in all treatment groups.
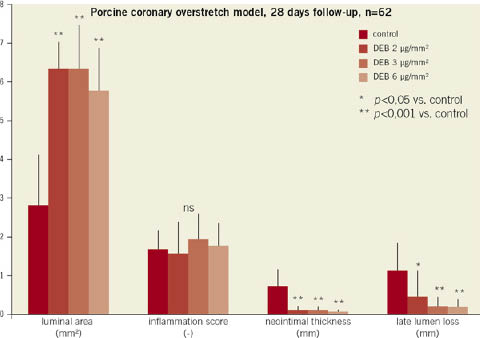
Figure 2. Results of histomorphometry and quantitative coronary angiography of stented porcine coronary arteries after 28 days.
In this preclinical animal study, paclitaxel-coated Paccocath balloon catheters were found to effectively reduce neointimal proliferation in a dose range ≥3 µg paclitaxel/mm2 balloon surface. Inhibition of neointimal proliferation did not significantly increase if higher doses were applied. However, overdosing proved to be safe within the tested range.
Very short-term exposure and overlapping
In our early preclinical trials we tested the highest paclitaxel dose which at that time could be coated on the balloons (i.e., about 3 µg/mm2). A lower dose was less efficacious.4,5 Similarly, one minute inflation time was selected as the longest possible time which can be routinely applied in coronary arteries.4,6,7 No data were available yet indicating efficacy and tolerance of higher doses and shorter inflation times. Meanwhile, the coating method was improved and higher doses could be deposited on the balloons. Also, overlapping of coated balloons may increase the local paclitaxel deposition in tissue. Because of the lack of data from animal experiments, overlapping of balloons was excluded in initial clinical trials.6
We were frequently asked whether shorter times are similarly efficacious. This could not be tested in patients before without deliberately accepting the risk of inefficacy. The same applies to the increased dose because of the potential risk of delayed healing and thrombotic occlusions.
The aim of another animal study therefore was to evaluate the influence of these two critical features of drug-coated balloon application - inflation time and increased dose due to overlapping balloons. We addressed these questions in the established porcine model of coronary overstretch and stent implantation.8
Conventional PTCA angioplasty balloon catheters (length, 20 mm; diameters 3.0 and 3.5 mm) were coated with paclitaxel to which was added a small amount of the hydrophilic x-ray contrast medium iopromide (Ultravist) in the same way as previously published using acetone as the main solvent.4 The dose was 5 µg paclitaxel/mm2 of balloon surface which resulted in a diameter of the folded balloons of 1.1 mm and 1.15 mm, respectively. After coating, uncoated bare stents were crimped onto the balloons.
Thin bare metal stents (diameters, 3.0 and 3.5 mm; length, 19 mm) were implanted with an oversize ratio of ≈1.2. Twenty-eight pigs received a total of 56 identical stents into the LAD and Cx arteries according to the randomisation list. The stents were premounted on identical balloon catheters, which were either uncoated or coated with a paclitaxel dose of 5 µg/mm2 of balloon surface. The animals were randomised to five different treatments: (1) control, consisting of bare stents crimped onto conventional, uncoated PTCA balloon catheters, 60 sec inflation time (n=14 vessels); (2) DCB 10s, consisting of bare stents crimped onto paclitaxel coated PTCA balloon catheters, 10 seconds inflation time (n=10 vessels); (3) DCB 60s, consisting of bare stents crimped onto paclitaxel-coated PTCA balloon catheters, 60 seconds inflation time (n=10 vessels); (4) DCB 2×60s, consisting of bare stents crimped onto paclitaxel-coated PTCA balloon catheters, 60 seconds inflation time and 60 seconds post-dilatation with the same balloon catheter, 30 seconds interval between inflations, serving as control for the next group (n=10 vessels); (5) 2×DCB 60s, consisting of bare stents crimped onto paclitaxel-coated PTCA balloon catheters, 60 seconds inflation time and 60 seconds post-dilatation with a second paclitaxel-coated (5 µg/mm2) balloon catheter shortly thereafter (n=12). After four weeks, the animals were sacrificed after follow-up angiography.
Paclitaxel balloon coating resulted in a marked and statistically significant reduction of late lumen loss and a statistically significant increase in minimal lumen diameter compared with controls (Figure3). Reduction of angiographic restenosis did not differ between treatments performed with different inflation times (10, 60, and 2×60 seconds) or dosages (5 µg, and 2×5 µg paclitaxel/mm2 balloon surface as used in the 2×DCB 60 second group). Histomorphometry showed a statistically significant increase in lumen diameter and luminal area and a corresponding decrease in maximal neointimal thickness and neointimal area in the vessels treated with paclitaxel-coated balloons. The DCB 10s treatment reduced the neointimal area by 57% compared to controls and the 2×DCB 60 second treatment, by 61%. Despite the marked reduction of neointimal proliferation, endothelialisation of stent struts was present in all samples. Injury scores were comparable between all groups. Inflammation scores were identical with the exception of the control group.
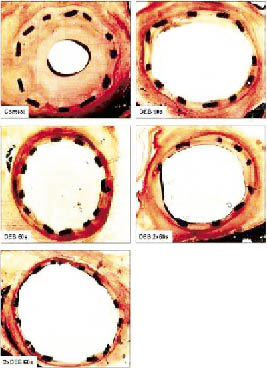
Figure 3. Results and representative examples of histomorphometry of stented porcine coronary arteries after 28 days.
The results presented here show that even very short-term exposure to paclitaxel-coated balloon catheters is sufficient to inhibit restenosis in the animal model. In the present study, efficacy of the DCB in combination with a modern, uncoated, thin bare metal stent was independent of inflation time. Furthermore, neointimal proliferation and all other parameters characterising in-stent restenosis could not be further decreased by inflating two DCB (each containing 5 µg paclitaxel/mm2 balloon surface) in the same vessel segment for 60 seconds each. These results suggest that the DCB releases most of the drug rapidly, during the first seconds of inflation.
Obviously, the initial contact of the coated balloon membrane with the vessel wall is sufficient to inhibit neointimal proliferation. Critically ill patients frequently do not tolerate prolonged ischaemia time due to long-lasting balloon inflation. The results of our study in pigs indicate that it may be sufficient to inflate the balloon for afew seconds only to achieve the desired protection from restenosis. Furthermore, long lesions could be treated by using several coated balloons with overlapping areas. Vessel segments treated with overlapping balloons will be exposed to a doubled dose. These animal data had to be provided to assess the risk of high local doses before initiating clinical studies that also allow use of overlapping treatment. In the current study, tolerance was established even for the highest dose delivered to a single vessel segment using paclitaxel-coated balloons. Adose of 10 µg/mm2 is more than 3 times the dose level applied in the treatment of in-stent restenosis in patients.6
In conclusion, the DCB seems to be a promising new tool to overcome some limitations of DES. The animal experiments indicate that only a very short inflation time of the balloon is required to achieve inhibition of neointimal proliferation. The increased local dose resulting from overlapping balloons seems to be well tolerated. It may therefore be possible to include patients at increased cardiovascular risk in future clinical trials.
Early endothelialisation and neointimal proliferation up to 6-months after implantation of BMS by paclitaxel-coated balloon catheters, Cypher and Taxus stents
Concerns have been raised that sustained drug release by drug-eluting stents (DES) may be associated with an increased incidence of late thrombotic complications because of delayed endothelialisation. In contrast, drug-coated balloon catheters (DCB) allow for rapid dilution and elimination of antiproliferative active substances after the short inflation time of the balloon. Neither the time course of healing nor the duration of stenosis inhibition has yet been investigated in a way which allows a comparison of effects following implantation of drug-eluting stents with the use of drug-coated balloons.
Another animal study by our working group investigated endothelialisation and neointimal proliferation in the porcine coronary overstretch model after implantation of DCB in combination with bare metal stents (BMS) for the first time compared to Cypher and Taxus DES.9
Domestic pigs were randomised to four different treatments: (1) control, consisting of uncoated bare metal stents crimped onto uncoated PTCA catheters (n=46 vessels); (2) DCB+BMS, consisting of uncoated bare metal stents crimped onto PTCA catheters coated with a paclitaxel dose of 3 µg/mm2 of balloon surface (n=50 vessels); (3) Taxus, paclitaxel-eluting stents (n=50 vessels); (4) Cypher, sirolimus-eluting stents (n=50 vessels). Oral aspirin 100 mg and ticlopidine 250 mg per day were administered until sacrifice.
After three and seven days, as well as after one, three and six months, quantitative angiography (QCA) and histomorphometry of the stented arteries was performed. After QCA, hearts were rapidly excised, the coronary system flushed with 0.9% saline and the arteries fixed by perfusion with 4% buffered formalin. The target segments were carefully dissected and samples for histology were obtained. To assess the endothelialisation score the tissue was stained by anti-von Willebrand factor (vWF) monoclonal antibodies before embedding.
There were no thrombotic complications and no other significant adverse events in the treatment groups during or after coronary interventions. Endothelialisation three days after the coronary intervention was similar in the DCB+BMS group compared to the uncoated control group, whereas it was delayed with the Cypher stent versus the control group (p=0.02) and DCB+BMS group (p<0.01). However, the difference between the Taxus group and control or DCB+BMS did not reach the level of statistical significance (>0.05).
The DCB+BMS treatment led to a highly significant reduction of neointimal formation after 28 days compared to control and both types of DES. As reported in previous studies in pigs, both DES showed accelerated neointimal formation after three and six months in spite of sustained drug release. In contrast, also after several months late lumen loss and neointimal formation in the animals treated with the paclitaxel-coated balloon were less pronounced compared to the animals treated with drug-eluting stents (Figure4).
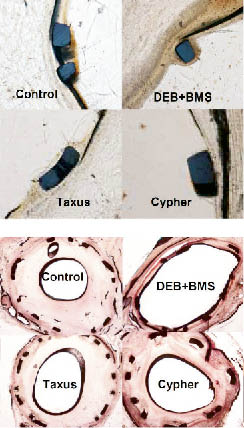
Figure 4. Results and representative examples of histomorphometry of stented porcine coronary arteries.
It may be concluded that efficacy of the paclitaxel-coated balloon in respect of the inhibition of excessive neointimal formation following vessel wall injury is at least as sustained as with the drug-eluting stents. According to the results of this study, the immediate release of the drug did not result in a shorter duration of the desired effect. In respect of the inflammation score, no consistent difference between the groups was detected.
The DCB combined with uncoated bare stents shows improved short-time (early endothelialisation) and long-term (pronounced inhibition of neointimal proliferation) features compared to drug-eluting stents in the animal model.
Comparison of two different paclitaxel-coated balloon catheters: PACCOCATH and DIOR
Paclitaxel-coated balloon catheters (DCB) based on the PACCOCATH matrix technology have shown excellent effects in the treatment and prevention of restenosis in the porcine coronary overstretch model,4,5,7-9 and in coronary in-stent restenosis in patients.6,10 The favourable results obtained so far suggest that the DCB has the potential to become established as the currently most advanced alternative to drug-eluting stents for local drug delivery.
The first generation DIOR balloon catheter has also been introduced for paclitaxel delivery in PCI. Although the paclitaxel dose is similar to that in the matrix coated Paccocath balloon, coating was done in a different way. Adherence of the drug to the balloon surface was mediated by roughening of the membrane. Few experimental and clinical data regarding safety and efficacy have been published. The aim of our preclinical study was to compare the safety and efficacy of PACCOCATH and the first generation DIOR DCB in inhibiting neointimal proliferation in the porcine coronary overstretch model.11
Bare metal stents (Bavaria Medizin Technologie, Oberpfaffen-hofen, Germany) were implanted with an oversize ratio of ≈1.2 (implantation pressure 10-12 atm, inflation time 60 seconds). Fourteen domestic pigs received a total of 28 stents (diameter 3.5mm, length 18mm) into the LAD and Cx arteries. The stents were premounted on either uncoated (control, n=11 vessels) or paclitaxel-coated PCI balloon catheters (Paccocath matrix-coating: n=8 vessels; roughened-surface coating, DIOR, Eurocor GmbH, Bonn, Germany: n=9 vessels). According to the manufacturers (Eurocor, DIOR product brochure, Rev. No. 0807 B7) the paclitaxel dose on the balloon surfaces was 3 µg/mm2 in each case. After inflation, stent deposition, and balloon withdrawal through the guiding catheter, the paclitaxel content of the balloons was determined by HPLC. After four weeks, the animals were sacrificed after follow-up angiography.
There were no acute thrombotic complications and no significant adverse events in terms of ECG parameters during or after the coronary interventions. After inflation, stent deposition and withdrawal of the balloon through the guiding catheter, the paclitaxel content of the balloons was measured. The postprocedural content was 50.4±11.5% of the initial dose (specified by Eurocor, DIOR product brochure) on the roughened DIOR balloon catheters (p<0.001 vs. control), and 4.5±0.7% on matrix coated Paccocath balloons (p<0.001 vs. control, p<0.001 vs. roughened DIOR DCB).
Treatment with the matrix coated Paccocath balloon resulted in a marked and statistically highly significant reduction of late lumen loss in-segment and highly significant increase in minimal lumen diameter compared to control (p<0.001) and the roughened DIOR DCB (p<0.001). Uncoated balloon PCI led to a late lumen loss of 1.85±0.52 mm (n=11) within the stented segments. Angioplasty with the roughened DIOR DCB failed to show a significant reduction in late lumen loss in-segment compared to control (1.41±0.48 mm, n=9). In contrast, late lumen loss in vessel segments treated with the matrix-coated Paccocath DCB was reduced to 0.43±0.20 mm (n=8, p<0.001 vs. control, p<0.001 vs. roughened DIOR DCB) 28 days after intervention.
After four weeks, histological evaluation showed that all stents were sufficiently expanded. Complete endothelialisation was present in all samples. Inflammation scores were similar in the different treatment groups. Corresponding to the angiographic findings, histomorphometry showed a statistically significant increase in lumen diameter and luminal area in the vessels treated with the matrix coated Paccocath DCB compared to control and the roughened DIOR DCB. In contrast, treatment with the roughened DIOR DCB resulted only in a slight decrease in neointimal hyperplasia, which was not statistically significant in comparison to the control group. Four weeks after intervention, uncoated balloon angioplasty led to a maximal neointimal thickness of 1.24±0.59 mm (n=11). Vessels treated with the matrix-coated Paccocath DCB displayed a highly significant reduction of maximal neointimal thickness to 0.42±0.14 mm (n=8, p<0.001 vs. control, p<0.01 vs. roughened DIOR DCB. However, neointimal thickness was not significantly reduced by the roughened DIOR DCB (0.86±0.37 mm, n=9). Neointimal area was highly significantly (p<0.001) reduced by treatment with the matrix-coated Paccocath DCB (2.48±0.78 mm2, n=8 vs. 5.72±1.51 mm2, n=11 (control)). In contrast, the roughened DIOR DCB reduced the neointimal area to only 4.05±1.65 mm2 (n=11), which reached the defined level of statistical significance (p=0.03 vs. control) (Figure 5).
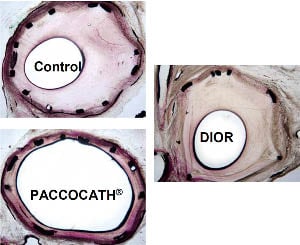
Figure 5. Results and representative examples of histomorphometry of stented porcine coronary arteries after 28 days.
As the matrix of a DES is at least as important as the antiproliferative drug itself, the same holds true for DCB coating. The challenge posed by drug-eluting balloons is to ensure adhesion of the drug on the balloon while transferring it to the target vessel segment. Upon inflation, which should be as short as possible, an effective dose has to be released to ensure transfer of a large enough amount of the drug into the vessel wall. In our initial animal studies, several coatings failed to cause sufficient neointimal inhibition.4 Finally, a specific matrix-coating (paclitaxel to which a small amount of the hydrophilic x-ray contrast medium iopromide, the iodinated component in Ultravist, was added), resulted in substantial neointimal inhibition in animals.4,7 These effects have since been confirmed in clinical trials.6,10 Matrix coating results in a porous layer with a large contact surface between the lipophilic drug molecules and the vessel wall. It provides uniform, reproducible and complete release of the drug after the first balloon expansion. These attributes result in a high bioavailability of paclitaxel at the target site, a precondition for rapid drug absorption by the vessel wall.4
In the present study, only the paclitaxel-coated Paccocath DCB based on the matrix technology led to a major reduction of neointimal proliferation in the porcine coronary overstretch model. The first generation DIOR balloon had a ‘nanoporous’ balloon surface. In contrast, the Paccocath matrix-coating uses small amounts of iopromide (Ultravist) as matrix to enhance the release and dissolution of the drug, possibly also the adherence to the vessel wall. Iopromide is used anyway as x-ray contrast medium in angioplasty. Only 30-45% of the drug-coating is released from roughened balloons during the recommended balloon inflation time of 45-60 seconds12. These findings are consistent with the results of the present study, showing a postprocedural paclitaxel content of 50.4% on balloons with comparatively high variance (SD 11.5%). In contrast, the iopromide matrix was found to release the full amount of the drug (4.5±0.7% of paclitaxel dose on balloons after the procedure), which may contribute to the superiority in restenosis inhibition.
Therefore, the favourable preclinical and clinical findings with the paclitaxel-iopromide coated Paccocath balloon cannot necessarily be generalised to other balloon coatings. Preclinical and clinical12 trials with paclitaxel on the roughened DIOR balloon membrane did not yet demonstrate efficacy in restenosis inhibition. Meanwhile, the first generation DIOR DCB was withdrawn from the market. This comparison demonstrates that the challenge with drug-coated balloons is to achieve fast release upon inflation, and persistent efficacy in restenosis prevention and safety without long-lasting double antiplatelet therapy.
Clinical evaluation of the drug-coated balloon
First proof of concept in man: PACCOCATH ISR I and II trial
Despite serious doubts in the efficacy of the single dose short-term treatment with drug-coated balloons, we started a randomised study in patients with coronary in-stent restenosis. The first patient in the PACCOCATH ISR I/II trial was enrolled end of December 2003.6,10
The Paccocath In-Stent-Restenosis (ISR)-I trial was a German, controlled, randomised, first-in-human, multicentre study with blinded angiographic evaluation that compared the efficacy and tolerance of Paccocath paclitaxel-coated balloon catheters with conventional uncoated catheters for the treatment of coronary in-stent restenosis. Compared to patients treated with an uncoated balloon, patients in the Paccocath balloon group had significantly better angiographic results (in-segment late luminal loss 0.74±0.86 mm versus 0.03±0.48; p=0.002) and concomitant 12-month clinical outcomes.6 The subsequent Paccocath ISR-II trial extends the initial findings from the first ISR trial.10 The second trial reproduced the ISR-I results in an additional 56 patients with similar baseline clinical and angiographic data. The most surprising finding was that the beneficial effects of the Paccocath balloon catheter were maintained for up to two years after the intervention.10 Importantly, in contrast to drug-eluting stents, combined antiplatelet therapy was continued for only one month, followed by treatment with aspirin alone. Figure6 shows a patient with treatment of an occluded stent in the Cx artery with the Paccocath balloon, including serial angiographic follow-up up to 5.5 years.
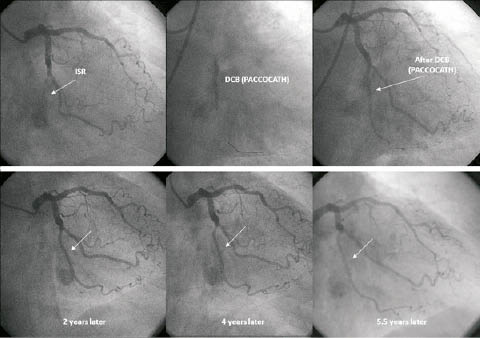
Figure 6. Angiographic long-term follow-up after recanalisation of a bare metal stent in the circumflex coronary artery by a drug coated Paccocath balloon.
Conflict of interest statement
B. Scheller reports receiving grant support from B. Braun; speaker honoraria from B. Braun and Invatec; major stock shareholder/equity in InnoRa GmbH; and named as co-inventor of a patent application submitted by Charité University Hospital (Berlin, Germany). B. Cremers reports speaker honoraria from B. Braun and Invatec.
References

