Introduction
Drug-coated balloons (DCBs) have been increasingly used because of their potential to combine balloon angioplasty and antiproliferative drug elution without leaving a permanent metal implant that may distort and constrain the coronary vessel, limit vasomotion and adaptive remodelling, and promote chronic inflammation. Additionally, the use of DCBs reduces the need for long-term dual antiplatelet therapy, which can increase the risk of bleeding. However, there is limited evidence from randomised controlled trials on the use of DCBs to treat patients with de novo coronary lesions, and concerns arise particularly when treating patients with complex lesions or high-risk clinical profiles. Whether the long-term safety and efficacy of DCBs will surpass that of the latest generation of drug-eluting stents in patients with de novo coronary lesions remains uncertain.
Pros
Antonio Colombo, MD; Pier Pasquale Leone, MD, MSc
The main reasons supporting the use of drug-coated balloons for patients with de novo lesions are the following:
Drug-eluting stent (DES) implantation gives an immediate, stable result. In some scenarios, DES implantation mitigates the risk of emergency bypass surgery, myocardial infarction and sometimes death; several randomised studies and registries endorse the first 2 points.
Nonetheless, are there any limitations to universal DES implantation when performing percutaneous coronary intervention (PCI)?
1) There is a 2% yearly attrition rate in adverse events following DES implantation, and this value increases when dealing with long stents as well as in diabetic patients;
2) Stents negate positive vascular remodelling and pulsatile function in the treated segment;
3) Stenting from healthy-to-healthy vessels, proposed at the time of short stents, leads to the implantation of very long stents, increasing the concerns named in (2);
4) A suboptimally implanted stent may be deleterious;
5) Sometimes stent delivery may be very complex;
6) Stent restenosis may be difficult to treat.
What is new in the interventional toolkit?
There is the new possibility to deliver an effective antiproliferative medication (paclitaxel or limus) utilising a drug-coated balloon without the need of a permanent metal device. We hypothesise that, if an adequate and stable lumen is obtained following lesion predilatation, the utilisation of a DCB will prevent restenosis without the need to implant a DES. We cannot deny that in many lesions the result after predilatation is optimal (less than 30% residual stenosis), and small type A or B dissections do not lead to vessel occlusion1. In these situations, DES implantation may not be necessary. We propose measuring the distance of the distal-to-aortic coronary pressure ratio (Pd/Pa) distal to the treated lesion to guide this strategy when dealing with an uncertain result (Figure 1)2; a residual lumen area of 5.5 mm2 or larger, as detected by intravascular ultrasound, (in a 3 mm vessel) may be needed to allow lumen preservation during vessel healing3. Several randomised studies and registries comparing DCB with provisional DES implantation versus routine stenting will provide answers.
If acted upon as expressed above, patient safety will not be compromised, and a leap forward in the efficacy of PCI may be obtained.
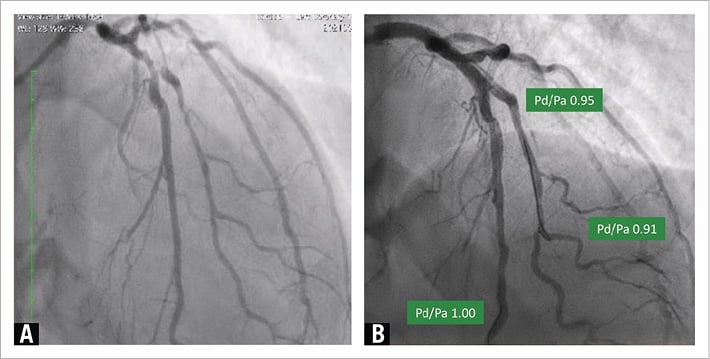
Figure 1. Pd/Pa-guided drug-coated balloon angioplasty. A) Critical lesions of the left main stem and the left anterior descending (LAD)-diagonal bifurcation. B) Immediate result after DES implantation on the left main stem and after lesion preparation with non-compliant balloons and DCB angioplasty on the LAD-diagonal bifurcation. A good angiographic result and Pd/Pa values >0.90 on the LAD and diagonal support a DCB-only approach, notwithstanding the NHLBI type B dissection on both vessels. DCB: drug-coated ballon; DES: drug-eluting stent; NHLBI: National Heart, Lung, and Blood Institute; Pd/Pa: distal-to-aortic coronary pressure ratio
Conflict of interest statement
The authors have no conflicts of interest to declare.
Cons
Clemens Von Birgelen, MD, PhD; Eline H. Ploumen, MD, PhD
Drug-coated balloons were developed as an alternative to DES for percutaneous coronary intervention. However, current evidence only supports the use of DCBs for specific indications such as in-stent restenosis or small vessel lesions. Only a single trial, the BASKET-SMALL 2, has published longer-term follow-up data for small vessel lesions (<2.75 mm), showing similar clinical outcomes for DES and DCBs at 3 years1. Another study, the PICCOLETO II trial, found no difference in clinical outcome at 12 months4. Nevertheless, in those trials, the repeated revascularisation rates for patients treated with DES in small vessel lesions were strikingly higher than in a randomised DES trial without routine angiographic follow-up5: 4.5% target vessel revascularisation in the BASKET-SMALL 2 trial and 5.6% target lesion revascularisation rate in the PICCOLETO II4 trial, as compared to a 1.7% target lesion revascularisation rate in an all-comers trial5. This raises the question of whether optimal DES implantation and post-dilatation techniques were applied in these small vessel lesions. In addition, in a trial with angiographic follow-up4, the oculostenotic reflex may have inflated the repeated revascularisation rate.
In de novo coronary lesions in vessels >2.75 mm, the use of DCBs is currently far from evidence based. Data about DCB treatment in such “larger” de novo lesions are very scarce. Moreover, there is a lack of sufficiently powered randomised comparisons with contemporary DES in all-comers trials, which comprise many elderly patients with diffuse coronary disease and with calcified target lesions that may be less suitable for treatment with DCBs. Actually, this is not surprising, as metallic coronary stents were initially developed for safety reasons, to treat – and thereafter, to prevent – major coronary dissections following balloon angioplasty, which, particularly in larger vessels, pose a substantial risk of morbidity and mortality. Yet, a small randomised study in 60 unselected patients observed similar clinical outcomes after treatment with DCBs versus second-generation DES at 8-month follow-up6. Furthermore, a meta-analysis of predominantly smaller randomised trials in selected patients (i.e., with myocardial infarction at presentation, high bleeding risk, small vessel disease or bifurcation treatment) found no difference in target lesion revascularisation rates between DCBs and DES7.
While the absence of a permanent coronary implant may sound appealing, to date, no large-scale randomised trial has demonstrated advantages in clinical outcome after DCB treatment versus new-generation DES implantation in de novo lesions in vessels of various sizes. Meanwhile, the safety and efficacy of contemporary DES in all-comer patients is well established, even at long-term follow-up. The data for DCBs in de novo lesions have yet to match that body of evidence. In the meantime, DES remain the mainstay of percutaneously treating de novo coronary artery disease.
Conflict of interest statement
The authors have no conflicts of interest to declare.

