- drug-eluting balloon
- clopidogrel
- drug-eluting stent
- coronary artery disease
Abstract
The high rate of restenosis associated with percutaneous coronary intervention (PCI) procedures can be reduced with the implantation of metallic stents into the stenotic vessels. The knowledge that neointimal formation can result in restenosis after stent implantation led to the development of drug-eluting stents (DES) which require long lasting antiplatelet therapy to avoid thrombotic complications. In the last years, the drug-eluting balloon (DEB) technology has emerged as an alternative option for the treatment of coronary and peripheral arteries. Clinical studies demonstrated the safety and effectiveness of DEB in various clinical scenarios and support the use of paclitaxel-eluting balloons for the treatment of in-stent restenosis, of small coronary arteries and bifurcations lesions. The protocols of DEB studies suggest that the dual antiplatelet therapy with aspirin and clopidogrel of four weeks after DEB is safe and effective.
Introduction
The prevention of restenosis has been one of the most important challenges since the beginning of the history of percutaneous coronary intervention (PCI). Many efforts have been put into pharmacological and interventional approaches for the prevention of restenosis. The advent of drug-eluting stents (DES) suppressing neointimal proliferation by sustained release of antiproliferative drugs had led to a dramatic change in the long term outcome of PCI, showing better results in comparison with bare metal stents (BMS) by reducing target lesion revascularisation (TLR) and binary angiographic restenosis1,2. Dual antiplatelet therapy (DAPT) with aspirin and clopidogrel has been proven to be very effective to prevent acute and long-term thrombotic complications after coronary stenting, for BMS and DES. As compared to BMS usage, the implantation of DES, however, is associated with late stent thrombosis and the need of a long-term dual antiplatelet therapy3. In recent years, drug-eluting balloons (DEBs) have emerged as a therapeutic alternative in the interventional field4. The concept of DEB uses a balloon catheter to deliver an antirestenotic drug, such as paclitaxel, at the site of arterial disease. Pre-clinical studies demonstrated effective inhibition of restenosis after PCI with stent implantation (ISR). Subsequent clinical trials successfully validated the efficacy of apaclitaxel coated balloon catheter to treat coronary in-stent restenosis after two years’ follow-up. This review summarises the current use of dual antiplatelet therapy in clinical studies with DEB and the current recommendations of the German “DEB only Consensus Group”.
Dual antiplatelet therapy in drug-eluting balloon studies
In pre-clinical studies, local paclitaxel delivery as a coating on a conventional PCI balloon has been proven to be successful in porcine coronary and peripheral arteries. In a porcine coronary artery study, Scheller et al5 have shown that after a 60-sec dilatation, most of the drug is released from the balloon (~90%). Even 40-60 min later they could detect 10-15% of the drug in the vessel wall, thus suggesting that paclitaxel is rapidly transferred from the balloon and retained by the tissue for a long time.
Clinical experience with DEB began with the treatment of in-stent restenosis by paclitaxel coated balloon catheters in the PACCOCATH ISR I trial6. This was a controlled, randomised blinded first-in-man study including 52 patients and comparing paclitaxel-coated balloon (PCB) catheters and standard plain old balloon angioplasty (POBA) for treatment of coronary in-stent restenosis. Patients treated with the coated PACCOCATH balloon (MEDRAD, INC., Warrendale, PA, USA) had significantly better angiographic results and concomitant 12-month clinical outcomes compared with patients treated with an uncoated balloon. The mean (±SD) in-segment late luminal loss was reduced from 0.74±0.86mm in the uncoated balloon group to 0.03±0.48 in the coated-balloon group (p=0.002). There were no coating related adverse events. Patients received a loading dose of 300mg of clopidogrel the day before the procedure or 600mg immediately beforehand. In the follow-up, patients received daily aspirin (100mg) and clopidogrel (75mg) orally for one month in both groups, followed by treatment with aspirin alone. Although clopidogrel was administered for four weeks only after drug-coated balloon in the PACCOCATH ISR trials, no thrombotic complications occurred during the two year follow-up period. Subsequently, these results have been augmented and confirmed by additional recruitment and longer follow-up in the PACCOCATH ISR II trial7 using the same protocol of DAPT.
Further data for this indication have been provided by a comparison of the SeQuent Please DEB (B Braun, Melsungen, Germany) with a paclitaxel-eluting stent in the Paclitaxel Coated Balloon versus Paclitaxel Coated Stent for the Treatment of Coronary In-stent Restenosis (PEPCAD II) study8. This study randomised 131patients with in-stent restenosis. At 6 months follow-up, in-segment late lumen loss was 0.38±0.61 mm in the DES group versus 0.17±0.42 mm (p=0.03) in the DEB group, resulting in a binary restenosis rate of 12 of 59 (20%) versus 4 of 57 (7%; p=0.06). At 12 months, the rate of major adverse cardiac events were 22% and 9%, respectively (p=0.08). Patients received 250 mg of aspirin intravenously before PCI and a loading dose of 300 mg of clopidogrel the day before the procedure or 600 mg immediately before the intervention. All patients received at least 100 mg of aspirin daily lifelong. Clopidogrel (75 mg/day) was given for three months after DEB angioplasty and for six months after DES implantation. There was no late thrombosis within the six month follow-up.
Treatment of lesions in small coronary arteries by PCI is limited by a high recurrence rate. In the PEPCAD I SVD trial9, 82 of 118 patients (70%) with a vessel diameter of 2.35±0.19 mm were treated with the DEB only, while 32 patients required additional stent deployment. The mean in-segment late lumen loss was 0.28±0.53 mm. In patients treated with the DEB only, the in-segment late lumen loss was 0.16±0.38 mm. At 12 months, the rate of major adverse cardiac events was 15% which was primarily due to the need for target lesion revascularisation in 14 patients (12%). In those patients with additional BMS implantation geographical mismatch between DEB dilatation and stent implantation was significantly associated with the occurrence of restenosis. Patients received 250 mg of aspirin intravenously and a loading dose of 300mg of clopidogrel the day before the procedure or 600mg immediately before the intervention. In the follow-up, all patients received at least 100 mg aspirin daily. Clopidogrel (75 mg/day) was given for one month following stand-alone DEB angioplasty, and for three months after additional BMS implantation. Two patients with additional BMS implantation presented with thrombotic occlusion of the stent two days and four months after the procedure, respectively. The patient with the subacute stent thrombosis was not under dual antiplatelet therapy. In both patients, the initial procedure was complicated by geographical mismatch between the DEB and the implanted BMS.
The PICCOLETTO trial10 employed a different paclitaxel eluting balloon technology not involving a drug carrier (Dior). This single centre trial enrolled a total of 80 patients with de novo small vessel (< 2.75 mm) lesions and randomised the patients to either the Dior DEB (Eurocor, Bonn, Germany) or to the Taxus Liberté DES (Boston Scientific Corporation, Natick, MA, USA). Enrolment was halted before completion due to the significant differences in outcomes seen between the groups. For the 57 patients analysed (6-month angiographic and clinical follow-up), the percent diameter stenosis (primary endpoint) was significantly higher in the DEB group (43.6%±27.4%) compared to the control group (24.3%±25.1%; p=0.029). All patients undergoing PCI received aspirin (either 100 mg/day for at least three days prior to PCI or a pre-PCI 300mg intravenous bolus), and clopidogrel (300 or 600mg as a loading dose, followed by 75mg daily). All patients continued aspirin indefinitely and clopidogrel 75mg daily for one month in cases of stable angina and lone PCB use, three months in cases of DEB and provisional stent implantation and 12 months in cases of unstable angina or Taxus implantation. There was no late thrombosis within the 9-months follow-up.
In comparison to non-bifurcated lesions PCI in bifurcation lesions is associated with lower immediate angiographic and clinical success and higher rates of restenosis11,12,13. The PEPCAD V trial14 enrolled 28 patients with bifurcation lesions in the left anterior descending artery/diagonal branch or the circumflex artery/obtuse marginal branch. The procedural success was achieved in all cases. The minimal lumen diameter increased from 0.80±0.39 mm to 2.56±0.44 mm in the main branch (p<0.001) and from 1.00±0.46mm to 1.87±0.35 mm in the side branch (p<0.001). Percent diameter stenosis declined from 73±13% to 15±9% (main branch, p<0.001) and from 59±19% to 23±11% (side branch, p<0.001). At nine months, re-angiography showed an in-stent late lumen loss of 0.38±0.46 mm, an 0.29±0.55 mm in-segment in the main branch resulting in a minimal lumen diameter of 2.2±0.60mm, an in-lesion late lumen loss of 0.21±0.48 mm and 0.12±0.47mm in-segment in the side branch resulting in a minimal lumen diameter of 1.7±0.44 mm. The patients underwent dual antiplatelet therapy with aspirin (100 mg/day) and clopidogrel (75 mg/day) for three months. Aspirin was continued thereafter. Two late stent thromboses were reported, one definite and one probable, at six and eight months after procedure. One of the two patients was a hypo-responder to both clopidogrel and aspirin.
The DEBIUT (Drug-Eluting Balloon in Bifurcation Utrecht) Registry15 evaluated the short-term safety and efficacy of a paclitaxel-coated balloon (Dior) in patients with bifurcation lesions followed by provisional stenting of the main branch. The procedure was successful in all patients. The use of sequential predilatation with DEB was safe and well tolerated. No acute or subacute closure of side branches occurred after treating with DEB. All patients were treated according to the provisional stenting technique; no stents were placed in the side branch. At the four month follow-up, no major acute coronary events and no subacute vessel closure were reported. All patients enrolled in the registry were treated with 325mg of aspirin and a 300mg loading-dose of clopidogrel 12 and two hours before the procedure, respectively. Aspirin was continued indefinitely after the procedure and clopidogrel (75 mg/day) for three months only. At four month follow-up no subacute vessel closure was reported.
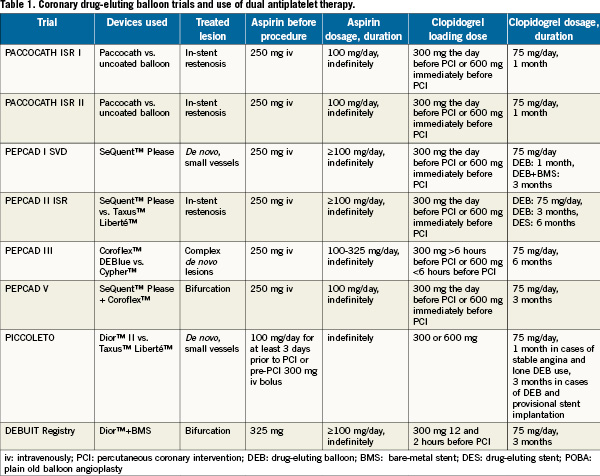
The use of dual antiplatelet therapy in coronary drug-eluting balloon is summarised in Table 1.
Recommendations for dual antiplatelet therapy after percutaneous coronary intervention with BMS OR DES
Clopidogrel is an inhibitor of ADP induced platelet aggregation acting by direct inhibition of adenosine diphosphate (ADP) binding to its receptor and of the subsequent ADP mediated activation of the glycoprotein GP IIb/IIIa complex. Combination therapy is particularly important in the patient receiving coronary stents. It is used after implantation of BMS for a minimum of 28 days peri- and post- procedurally to lower the incidence of acute (24 hours) and subacute (one to 30 days) stent thrombosis16. Randomised clinical trials have shown that drug-eluting stents reduce the risk of in-stent restenosis and the need for repeat revascularisation as compared with bare metal stents17. But DES also delay re-endothelialisation, which may predispose some patients to an increased risk of late stent thrombosis, although overall rates of stent thrombosis are similar between DES and BMS18.
Instructions for the use of drug- eluting stents specify treatment with clopidogrel for at least three months (for sirolimus coated stents) or six months (for paclitaxel coated stents) after implantation. A consensus panel review recommended in 2007 adding a minimum of 12 months of clopidogrel therapy to lifelong aspirin therapy19. Current clinical guidelines recommend a year of dual antiplatelet therapy for all patients treated with coronary stents20.
Potential risks related to clopidogrel therapy after stenting
A meta-analysis of 18 randomised trials comprising 129,314patients reported, that DAPT is associated with a significantly increased risk of major (RR 1.47, CI=1.36-1.60) and minor bleeding events (RR 1.56, CI=1.47-1.66) compared to single agent therapy21.
Despite significant benefits demonstrated with combination antiplatelet treatment in large clinical trials, the occurrence of adverse ischaemic events, including stent thrombosis, remains a serious clinical problem. Several studies have demonstrated distinct response variability and non-responsiveness to clopidogrel therapy based on ex vivo platelet function measurements. Small scale investigations have suggested that non-responsiveness may be associated with a heightened risk for adverse clinical events.
Furthermore, antiplatelet therapy may be stopped at the instruction of physicians, dentists or other health care providers because of misguided concerns about excessive procedure-related bleeding when they need to perform an invasive or surgical procedure on the patient. The premature discontinuation of DAPT is associated with a marked increase in the risk of stent thrombosis, and is the leading independent predictor for stent thrombosis in multivariate analyses22. In a large observational cohort study of patients treated with DES, stent thrombosis occurred in 29% of patients in whom antiplatelet therapy was discontinued prematurely23. Spertus et al, in the PREMIER (Prospective Registry Evaluating Myocardial Infarction: Events and Recovery) registry of 500 patients with acute MI treated with DES, reported that the mortality rate over the next 11 months of those who stopped thienopyridine therapy was 7.5% as compared with 0.7% in those who had not stopped therapy (hazard ratio 9.0, p<0.0001)24.
A relevant proportion of patients receiving aspirin and clopidogrel after PCI also require oral anticoagulation with a coumarin derivative such as phenprocoumon. Sibbing et al reported that phenprocoumon significantly attenuates the antiplatelet effects of clopidogrel25. Gilard et al evaluated the safety and efficacy of dual antiplatelet therapy in association with oral anticoagulant therapy in patients undergoing PCI. Severe and moderate bleeding, according to the Global Use of Strategies to Open Coronary Arteries criteria, occurred in 2.1% of patients when oral anticoagulation therapy was discontinued (22±31 days) and in 6.4% of patients when triple therapy was continued26.
Recent joint consensus guidelines from the American College of Gastroenterology (ACG), the American College of Cardiology (ACC) and the American Heart Association (AHA) endorsed the use of proton pump inhibitors (PPI) in patients judged to be at higher risk for gastrointestinal (GI) ulceration and related complications27. However, a number of other recent studies have revealed possible complications when clopidogrel and a PPI are used together and the European Medicines Agency (EMEA) also issued a statement that concomitant use of PPIs and clopidogrel is not advisable unless absolutely necessary, due to concerns that PPIs may reduce the effectiveness of clopidogrel28.
In practical terms, this means that risk stratification of long-term DAPT should be part of the interventional procedure strategy.
Recommendations for antiplatelet therapy by the German DEB only Consenus Group
Treatment of in-stent restenosis: The DEB is indicated for any ISR as a substitute for plain old balloon angioplasty (POBA) and as an alternative to DES to avoid a second stent layer. In case of treatment of an ISR with DEB the German DEB only “Consensus Group” recommends aspirin 100mg at midday long-term and clopidogrel 75mg at midday for four weeks after PCI in BMS and four weeks plus the additional DAPT mandated by the DES implantation date.
Treatment of small vessel disease: After treatment of de novo coronary lesions with reference diameters from 2.0 to 2.75mm aspirin 100 mg should be given at midday long-term and additional clopidogrel 75mg at midday is recommended for four weeks after PCI with DEB alone and for 6 to 12 months after DEB with additional BMS.
Treatment of bifurcation stenosis: if only DEB without stenting is used for the treatment of a bifurcational lesion, four weeks DAPT is recommended. DAPT is recommended for 12months due to the elevated stent thrombosis risk in bifurcational lesions and the DEB then BMS procedure.
Conclusions
The DEB represents an excellent therapeutic concept showing promising results in the treatment of coronary in-stent restenosis, of small vessels and coronary bifurcations. Beside the proven efficacy the possible reduction of DAPT for a duration of one month may represent an additional advantage regarding safety, patient compliance and costs. Concerning the new generation inhibition of platelet activation and aggregation, such as prasugrel and ticagrelor, data from studies with DES have to be awaited. These results may influence future recommendations of DAPT after DEB.
Conflict of interest statement
Dr. Bonaventura is a consultant to B.Braun. Dr. Sonntag has no conflicts of interest to declare. Dr. Kleber has received research grants from B.Braun, Invatec and Biotronik and is a consultant to B.Braun.
References

