- drug-eluting balloon
- bifurcation
- angioplasty
Abstract
Coronary bifurcation lesions, which account for 15-20% of all lesions treated percutaneously, remain hampered by procedural difficulties, post-procedural complications and suboptimal long-term results, even with the introduction of the drug-eluting stent (DES). Side branch (SB) restenosis rates remain a drawback even in the provisional T-stenting technique with final kissing balloons. The introduction of drug-eluting balloons (DEB) creates a new hope for this technique by maintaining the relatively easy provisional T-technique but promising better long term outcomes for the SB treated with DEB. The DEB delivers locally a high concentration of an anti-restenotic drug, paclitaxel, thereby potentially reducing restenosis rates as compared to aregular balloon. However little is still known on the optimal use and long-term outcomes of DEB in bifurcations. First results of the DEBIUT study will help to understand future directions in development of this new and promising device.
Rationale of the drug-eluting balloon in bifurcations
Coronary bifurcation lesions make up 15-20% of all percutaneous coronary interventions.1 This subset of lesions are considered as complex lesions, with high procedural costs and inferior angiographic and clinical results at follow-up.2 Several treatment strategies have been considered in order to improve outcomes. The use of a bare metal stent (BMS) resulted in better acute angiographic outcomes, however, this was accompanied with high rates of restenosis and still poor long-term clinical outcome.3 The introduction of drug-eluting stents (DES) showed in general a reduction in restenosis compared with BMS. However, the initial promising results of DES were not reproducible in the special circumstances of bifurcation lesions as the rates of restenosis, mainly in the side branch (SB), remained higher than in non-bifurcation lesions.4 Besides, the potential (increased) risk of stent thrombosis5 should be kept in mind whilst doing bifurcation stenting with DES since recently it has been shown that compliance to double antiplatelet therapy (DAPT) is low.6 Considering the fact that DES implantation and multiple stenting in bifurcation lesions warrants prolonged DAPT, the search for alternative treatment strategies such as drug-eluting balloons (DEB), with reduced DAPT seems appropriate.
Laboratory results showed that even a short contact between taxol compounds and vascular smooth muscle cells can inhibit the proliferation of the cells for a long period, so a stent-driven sustained drug release does not seem to be necessary at all.7 This was confirmed in animal experiments, where paclitaxel was delivered into coronary arteries using a contrast medium or a paclitaxel-coated balloon catheter. Both methods significantly reduced neointimal proliferation with a more pronounced reduction in the paclitaxel-coated balloon group.8 These findings were confirmed in clinical studies for the treatment of in-stent restenosis (PACOCATH ISR I and II, and PEPCAD II).9-11 The Paccocath ISR I and II trials were the first to demonstrate a significant reduction in the incidence of restenosis by using the Sequent Please paclitaxel-coated balloon compared with percutaneous coronary intervention with a normal balloon.
The potential advantages of the use of a DEB in bifurcations are:
1) homogeneous administration of the drug, whereas the DES only delivers the drug in the proximity of the struts;
2) delivery of high concentrations of drug into the vessel wall at the moment of injury;
3) respecting the original anatomy of the carina of the bifurcation;
4) avoidance of crushing of polymers and uncontrolled drug release (in case of two DES);
5) potential decrease in DAPT with reduced late thrombosis due to absence of polymers.
After successful completion of a pilot study,12 a physician initiated prospective, randomised, multicentre trial, comparing the outcome of DIOR DEB treatment in main and side branch with subsequent BMS implantation in the main branch with two control groups was initiated and finished enrolment early 2010 (ClinicalTrials.gov number, NCT00857441).
The drug-eluting balloon in bifurcations: current state of art
The Drug-Eluting Balloon in BIfUrcations Trial (DEBIUT) is a randomised multicentre single blind study, aimed at comparing a provisional T-stenting technique for coronary bifurcation lesions with pre-dilatation using DEB followed by BMS implantation (groupA), versus standard BMS implantation (group B) versus standard DES implantation (group C).
The main inclusion criteria were stable or unstable angina pectoris or silent ischaemia, due to de novo coronary artery lesions (stenosis >50% and <100%) at the level of a bifurcation. Eligible patients were assigned to one of the three treatment groups, with all three groups using a stent with the same design in order to exclude this confounding factor. A BMS stent was implanted in the main branch (MB) in groups A and B, after predilatation of both MB and SB with respectively DIOR DEB (Eurocor GmbH, Bonn/Germany) or regular balloon. In group C, a paclitaxel DES was implanted after dilatation with a regular balloon of both MB and SB. Final kissing balloon dilatation with regular balloons was mandated in all groups (Figure1). After the procedure patients were treated with three months of DAPT in groups A and B, or 12 months of DAPT in group C. A control coronary angiography was planned at 6-month follow-up. Major adverse cardiac events (MACE: death, myocardial infarction, target vessel revascularisation) were recorded up to 12 months. The primary endpoint was 6-month angiographic late luminal loss and the power analysis was based on the angiographic superiority of group A (DEB) over group B (BMS).
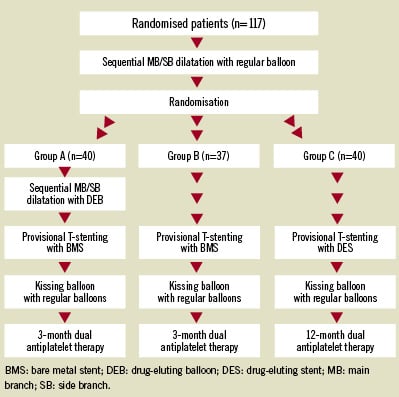
Figure 1. Flow-chart of the consecutive procedures in the three groups.
Overall, 117 patients (group A: 40; group B 37; group C 40) with suitable bifurcation lesions were treated according to the protocol. A total of eight (6.8%) periprocedural non-Q wave myocardial infarctions occurred during the initial hospitalisation without differences between groups. No further in-hospital MACE occurred. Considering the primary endpoint, group A showed a numerically similar late luminal loss as group B. The values of late luminal loss in group C were numerically and statistically better as both other groups. Binary restenosis rates per bifurcation and MACE rates were 24.2%, 28.6%, and 15% (p=0.45) and 20%, 29.7%, and 17.5% (p=0.40) in groups A, B and C, respectively. Summarising the main findings of the DEBIUT study:
1) the DIOR-I DEB failed to demonstrate angiographic superiority as compared to BMS, with similar late luminal loss and binary restenosis rates in both treatment groups;
2) DES showed better angiographic results than both DEB and BMS;
3) the reduced duration of dual antiplatelet therapy to three months appeared to be safe in combination with DEB and BMS.
Perspectives for future studies
Initially in the DEBIUT registry, 20 patients with bifurcation lesions were treated with a DEB, showing promising results with no MACE at 4-months of follow-up.12 Based on these promising findings, a larger randomised DEBIUT study was initiated. Clearly the initial promising results, as found in the DEBIUT registry, did not hold in the randomised DEBIUT study. One of the explanations for these discouraging results may be the technical properties of the DEB device. In this study the first generation DIOR-I was used. This device is coated with 3 micrograms of paclitaxel per square millimetre of balloon surface using a crystalline coating method with paclitaxel and dimethylsulfoxide. The un-inflated three-fold balloon protects the drug from an early wash-off effect during insertion into the guiding catheter, during tracking in the coronary vasculature and during crossing of the lesion. The drug delivery dose into the vessel wall is between 20-25% with inflation times between 30-60 seconds. According to animal data, this leads to a tissue dose of up to 6µg/mm2, while known data from Sequent Please have shown a tissue dose of up to 94µg/mm2.13 This indicates that even with comparable loading doses of 3µg/mm2, the delivery dose might differ significantly between DEBs.
With tissue delivery dose and release kinetics being the only variables among the three treatment groups in the DEBIUT study, our findings suggest that the pharmacokinetics of paclitaxel with DIOR-I may have been insufficient to provide comparable benefits, in terms of late luminal loss, to those observed in the DES arm. Hence, the use of a second generation, higher tissue delivery dose, DIOR-II DEB in combination with a new-generation BMS, might lead to better angiographic and clinical outcomes when used in bifurcation lesions. Although the results were not as promising as expected, the DEBIUT study provided some important findings:
1) feasibility of the use of a DEB in bifurcation lesions;
2) safety, with no occurrence of stent thrombosis during 12 month follow-up with just 3-months of DAPT;
The DEBIUT clearly leaves room for a second study using higher delivery dose drug-eluting balloons combined with either a new generation thin strut cobalt chromium stent14 or second/third generation DES with biodegradable polymers in order to prevent prolonged DAPT. Thorough research (i.e., fundamental, animal studies and clinical studies) should still be performed, in order to optimise this relatively new technique.
Conflict of interest statement
P. R. Stella is member of a Scientific Advisory Board of Eurocor GmbH. All other authors have no conflicts of interest to declare.
References

