Introduction
The drug eluting balloon (DEB) has demonstrated safety and efficacy for treatment of restenosed and de novo lesions in coronary artery disease in several clinical trials. Late lumen loss at follow-up is consistently low (~0.2mm), and no thrombotic event has been reported when using the DEB (Sequent®Please) as a stand-alone therapy.
Some issues remain when combining the DEB with a bare metal stent (BMS), since geographic mismatch (DEB does not cover total stented area) between DEB and BMS can not always be avoided. The combination of the DEB with a BMS further results in a somewhat higher late lumen loss comparable to paclitaxel eluting stent.
Summary of late lumen loss in PEPCAD trials
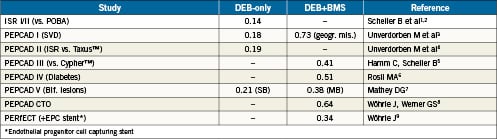
The DEB may be a valuable adjunct for indications where drug-eluting stents (DES) have limitations. It may also provide a new option for stand-alone balloon angioplasty. Although interventional cardiologists are very familiar with plain old balloon angioplasty (POBA), there are some questions specifically pertaining to DEB, for example:
How can we identify patients who may benefit from a DEB?
How should we proceed technically with the DEB?
A consensus group was formed to clarify these questions. The consensus group developed recommendations on the basis of available studies and on the basis of a consensus agreement, where no such studies were available. The group realised that this approach was not perfect, but it may be the “best of all possible worlds”. The expert panel members are listed in the appendix. Recommendations were first reported at Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium in 201010.
In March 2009, J. Wöhrle initiated a DEB registry11 to observe how the DEB (SeQuent®Please, B. Braun, Berlin, Germany) is used in everyday clinical practise. Until June 2010, 2,319 procedures have entered the registry, which showed that the DEB was mainly used in three indications: 62% in in-stent restenosis (ISR); 23% in small coronary vessels; and 13% in bifurcation lesions.
In-stent restenosis (bare-metal stents)
Scientific evidence
In PEPCAD II4, 131 patients with ISR after bare-metal stent (BMS) implantation were randomised to either receive a DEB or a Taxus stent.
Late lumen loss at six months, the primary endpoint of the study, was significantly smaller, 0.17mm, in the DEB group as compared to 0.38mm in the Taxus group. MACE at 12 months was 9% in the DEB group and 22% in the Taxus group, mainly driven by a TLR of 6% in the DEB group and 15% in the Taxus group.
These results show that the DEB was not only not inferior, but apparently, even superior to a DES in the treatment of ISR. It is for this reason that the European Society of Cardiology has given the DEB aclass IIa and level B recommendation for the treatment of ISR.12
Consensus group (Figure 1)
Predilation is considered mandatory in all cases. In order to avoid balloon slippage, a non- or semi- compliant balloon with a diameter of 0.5mm smaller than the reference diameter is advisable.
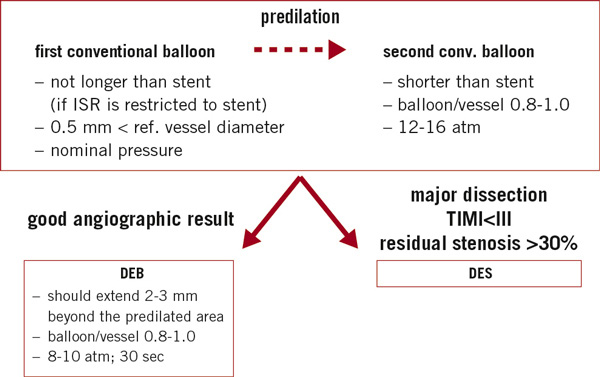
Figure 1. Treatment recommendations for in- stent restenosis.
Then, the use of a larger conventional balloon with a balloon-to-vessel ratio of 0.8-1.0 is strongly encouraged, particularly, if incomplete stent expansion is still visible. Cutting balloons, scoring balloons or non-compliant high-pressure balloons can also be taken into consideration.
After predilation, the operator has to decide whether to proceed with a DEB or whether to implant a DES in case of an extensive or flow-limiting dissection or a significant residual stenosis.
In case of a good angiographic results, a DEB can be used. It should extend beyond the pre-dilated area by 2-3mm on each side. It should also have a balloon to vessel ratio of 0.8-1.0 and be inflated for at least 30seconds at nominal pressure (about 8atm) to avoid dissection outside the stent.
In general, the DEB should not be used for direct mechanical treatment of in-stent restenosis, but rather as a device for drug-delivery after optimal predilation.
Lesions in small coronary arteries (2.25-2.75 mm)
Scientific evidence
Treating lesions in small coronary vessels was the second largest indication for the DEB, 23%. The indication is based on the PEPCAD I registry3. One hundred and fourteen patients with lesions in small coronary arteries (2.25-2.8 mm) were treated with a DEB and a BMS, when needed. Re-angiography was performed at 6-months. In 82 patients, only a DEB was used. In these patients, late lumen loss was 0.16 mm. In 32 patients, a BMS had to be implanted following the DEB. In these patients, late lumen loss was 0.62 mm. As a result, angiographic restenosis was seen in 13 of the 32 DEB/BMS patients. In 10 of the 13, geographic mismatch was noted.
Consensus group (Figure 2)
The consensus group recommended that lesions in small coronary arteries with a reference diameter of 2.0-2.75mm should be predilated with a conventional balloon. Again, the balloon/vessel ratio is 0.8 to 1.0. In case of a significant residual stenosis, a high pressure balloon may be considered. If the angiographic result is good, the DEB is used. It should extend beyond the predilated area by 2-3mm on each side, have a balloon/vessel ratio of 0.8-1.0, and be inflated at nominal pressure for a minimum of 30 seconds.

Figure 2. Treatment recommendations for small vessel disease.
In case of a significant dissection and/or a residual stenosis of ≥30% after predilation, the operator may proceed with a DES or consider DEB and BMS stenting. Only dissections type C-F require stenting.
Bifurcation lesions
Scientific evidence
In 13% of the DEB registry patients, a DEB was used for the treatment of bifurcation lesions. Bifurcation lesions are often difficult to treat and more prone to complications. Using the DEB may help to simplify the intervention.
The DEB was tested in bifurcation lesions in a small observational study with nine month angiographic follow-up (PEPCADV7). The main branch (MB) was treated with a DEB followed by BMS, the side branch (SB) with a DEB only in the majority of patients. As expected, the minimal lumen diameter in the main branch increased markedly after DEB and BMS stenting. After nine months, a late lumen loss of 0.38 mm was seen in the MB. In the side branch, where the DEB was mostly used without stenting, late lumen loss was only 0.21 mm, a DES-like result.
Consensus group (Figure 3)
The group recommended that predilation of the MB and SB was necessary in all cases. The conventional balloons used for predilation should have a balloon to vessel ratio of 0.8-1.0, and a length corresponding to the length of the stenosis.
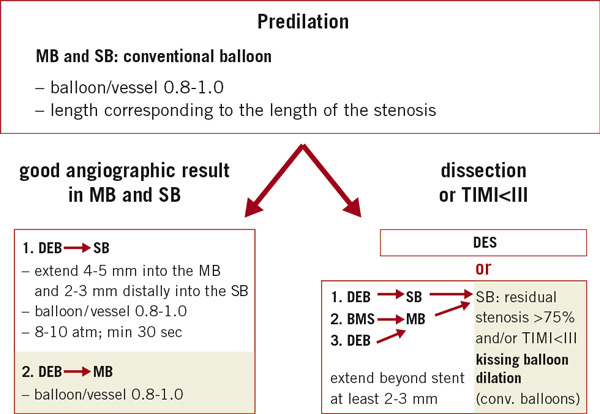
Figure 3. Treatment recommendations for bifurcation lesions.
After predilation, the operator has to decide to either proceed with a DEB, in case of a good angiographic result in the MB and the SB, or to proceed with stenting, in case of significant dissection or a reduced flow.
In case of a good angiographic result in the MB and the SB the SB is dilated with a DEB which should extend 4-5 mm into the MB and 2-3 mm distally beyond the PTCA area into the SB. The DEB balloon to vessel ratio is 0.8-1.0, and the DEB is inflated at nominal pressure. Even if the SB is not severely stenosed, a DEB treatment is recommended to prevent ostial restenosis after MB stenting. Then, the MB is dilated with a DEB, 0.8-1.0 balloon to vessel ratio. No stent is implanted.
In case of major dissection after predilation of the MB, the procedure can take its normal course, which means a DES is used.
Alternatively, the DEB can be used in combination with a BMS. In this case, the SB is dilated with a DEB. A BMS is then implanted in the MB followed by a DEB in the MB. The stent can also serve as a marker to avoid geographic mismatch. The DEB should extend beyond the predilated and stented segment by at least 2-3mm on each side.
If the SB has <75% residual stenosis and a TIMI 3 flow, no further treatment is deemed necessary. If the residual stenosis in the SB is >75% or TIMI flow is reduced, a final kissing balloon dilation with conventional balloons is recommended.
Dual antiplatelet therapy
Dual antiplatelet therapy (DAT) is necessary for four weeks if the DEB is used as a stand-alone procedure. In combination with a BMS, 6-12 months of DAT is recommended.
(If there is an indication for a longer period of DAT, for example the patient has received a DES within the last few months, than dual antiplatelet therapy has to be prolonged according to this latter indication.)
General principle
The general principle underlying the consensus group recommendations for in-stent restenosis, lesions in small coronary arteries and bifurcation lesions is illustrated in Figure 4. The lesion is always predilated with a conventional balloon, which has a balloon/vessel ratio of 0.8-1.0 to reach the final result.

Figure 4. General principle of recommendations.
Then the operator can decide to proceed with a DEB-only strategy in case of an acceptable angiographic result. The DEB length should always exceed conventional balloon length to completely cover total PCI area. In case of a major dissection, significant residual stenosis or reduced flow, a DES or DEB plus BMS should be implanted.
The consensus group realises that it is not easy for today´s interventional cardiologists to finish a coronary intervention without stenting. However, taking into consideration the necessity of bailout stenting in 5-8 % of patients, there is room for DEB as an alternative concept to reduce the need of stents.
The consensus group hopes that its recommendations will raise awareness for an approach that may help to improve and simplify catheter based coronary interventions.
Appendix
Members of the German consensus group
Bruch L, Berlin; Hengstenberg C, Regensburg; Kleber FX, Potsdam; Kurowski V, Lübeck; Mathey DG, Hamburg; Missler J, Stade: Moebius-Winkler S, Leipzig; Radke P, Lübeck; Rittger H, Coburg; Scheller B, Homburg/Saar ; Schneider H, Rostock; Zeus T, Düsseldorf; Zeymer U, Ludwigshafen
Conflict of interest statement
F.X.Kleber has a consulting agreement with B. Braun and has received lecture honoraria and free scientific research grants from B. Braun, Eurocor, Invatec-Medtronic and Biotronik. D.M.Mathey has received speaker fees from B. Braun. H. Rittger reports speaker honoraria from B. Braun. B.Scheller reports receiving grant support from B. Braun; speaker honoraria from B. Braun and Invatec; major stock shareholder/equity in InnoRa GmbH; and is named as co-inventor of a patent application submitted by Charité University Hospital (Berlin, Germany).
References

