Local drug release into the vessel wall is currently the dominant prevention strategy of restenosis1. Drug-eluting stents (DES) have become the mainstay of current revascularisation practice since they dramatically reduced the need for re-intervention as compared with bare metal stents (BMS)2. The superiority of DES over BMS has been demonstrated in various lesion settings,3 such as de novo atherosclerotic disease of native and graft vessels4 and in-stent restenosis (ISR) after BMS5 or DES implantation6. The platform, polymer and antirestenotic drug are the most common components of DES. Although the stent platform has been shown to have an important impact on the BMS performance7,8, its specific role as a component of DES has not been investigated. Findings from several studies support a relevant role of the other two DES components, the drug carrier and the antiproliferative drug3,9. There is sufficient evidence to show that drugs of the “limus” family are safer and more effective than paclitaxel3. Drug-eluting balloons (DEB) have emerged as a new PCI option10. Main DEB components are vehicles for drug-elution (carriers/excipients) and the antiproliferative drugs. Indeed, it has previously been demonstrated in animal models that antiproliferative drugs could be adequately delivered from balloon surface into the vessel wall only with a matrix compound11. Similarly, Radke et al compared various DEB using different drug-delivery formulations and confirmed that effective excipients are necessary to accomplish successful tissue transfer of the drug from a balloon surface12. Importantly, in vitro and in vivo models showed that during angioplasty with DEB, contact between vascular smooth muscle cells and paclitaxel, a highly lipophilic anti-neoplastic drug, is sufficient to provide long-lasting inhibition of cell proliferation13-15. As a result, paclitaxel became the sole drug used among DEB currently available on the market, with other drugs still under investigation16. It remains to be seen whether refinement of methods of drug delivery from balloons will enable effective use of “limus” drugs for DEB in the hope to repeat the success they have achieved as component of DES.
A discrete number of randomised studies evaluated DEB versus plain balloon angioplasty (POBA), DES and BMS in different settings17. The main limitation of these trials was the lack of power to assess clinically relevant endpoints. In Figure 1 we summarised risk estimates for DEB regarding target lesion revascularisation (TLR) by performing a meta-analysis of currently available randomised trials.
The main results are:
1) In de novo lesions, the combination of DEB with BMS, which makes little sense considering the widespread DES availability, does not reduce the need for re-intervention as compared with BMS alone and increases TLR risk as compared with DES (Figure 1, Panel A and B).
2) The size of the two trials18,19 that assessed DEB for lesions in small coronary vessels is limited and does not enable firm conclusions. However, no sign of benefit of DEB versus DES could be observed (Figure 1, Panel C). Randomised trials of BMS versus POBA in small vessels have shown that optimal POBA with provisional stenting is probably as effective over the long term as systematic stenting20. However, considering the fact that the efficacy and strength of current DES technologies is mostly generated when they are placed in lesions of small vessels, it is unlikely to expect a major role for DEB in the treatment of lesions in coronary vessels of small size21.
3) With less than 400 patients enrolled in total, randomised trials on the value of DEB for ISR showed that DEB compare favourably with POBA or paclitaxel-eluting stents reducing TLR risk in patients presenting BMS or DES restenosis22-25 (Figure 1, Panel D). In line with this evidence, the current guidelines for myocardial revascularisation of the European Society of Cardiology recommend the use of DEB in cases of restenosis of previously implanted BMS (Class of recommendation: IIa; Level of Evidence B)26.
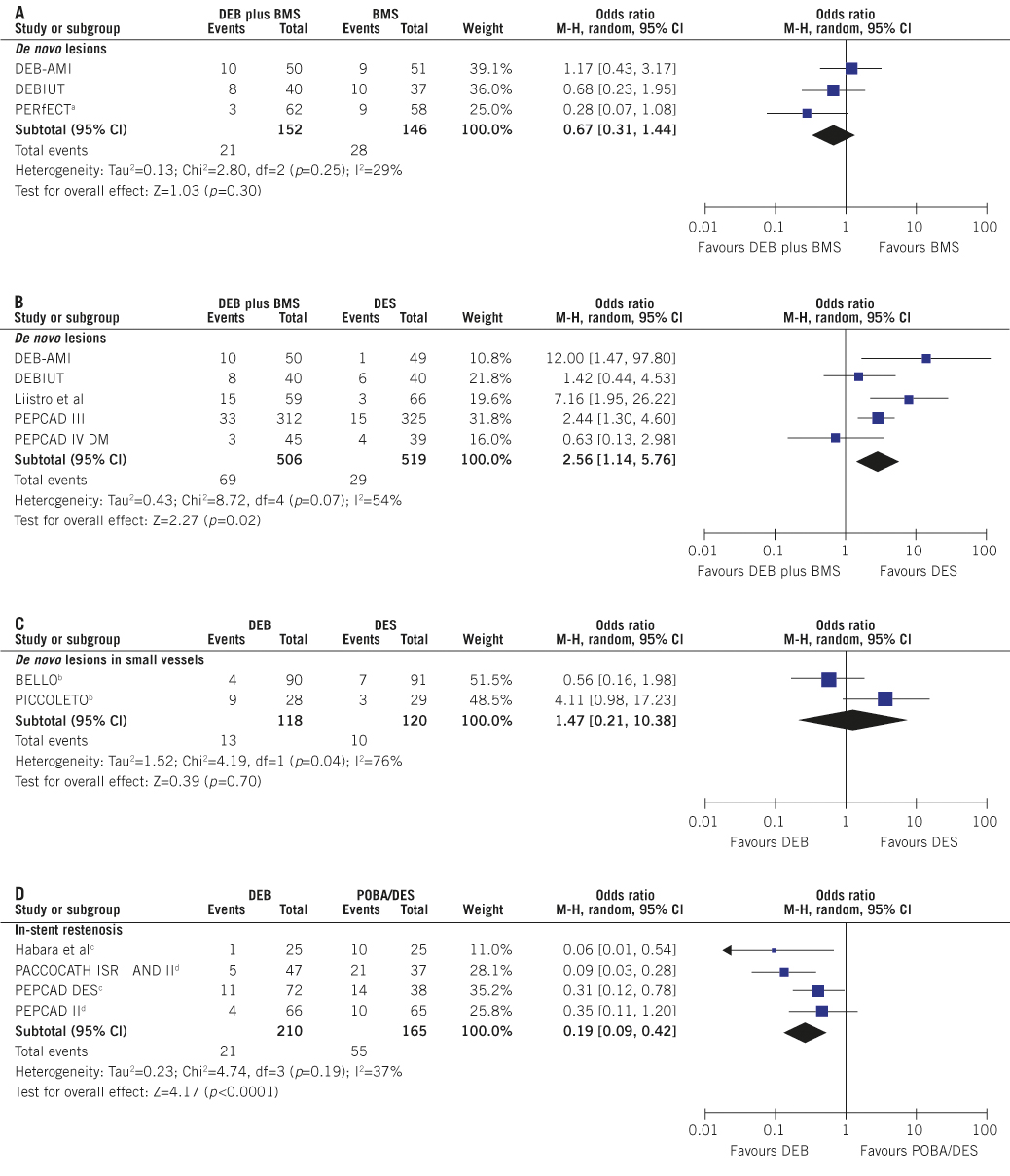
Figure 1. Risk of target lesion revascularisation associated with drug-eluting balloons. Odds ratio (OR) and (95% confidence intervals, CI) are used as summary statistics. DerSimonian and Laird random effects model is used to calculate pooled OR for target lesion revascularisation (TLR). The Breslow-Day chi squared (p<0.1) and the I2 statistic tested heterogeneity across the studies. The squares and the horizontal lines indicate the OR and the (95% CI); the size of each square is proportional to the statistical weight of a trial in the meta-analysis; diamond indicates the effect estimate derived from meta-analysis, with the centre indicating the point estimate and the left and the right ends of the (95% CI). In case of a three-arm design, the pooled risk estimates have been derived by separate comparison of DEB versus each control arm. The risk of TLR (per protocol defined) was evaluated at the longest follow-up (median 9 months [25th, 75th percentile 6, 12]) within de novo lesions (Panel A-B), de novo lesions in small vessels (Panel C) and in-stent restenosis subgroups (Panel D). Moderate to high heterogeneity was observed.
DEB: drug-eluting balloons; BMS: bare-metal stents; DES: drug-eluting stents; POBA: plain-old balloon angioplasty. a bioengineered bare-metal stent used; b provisional bare-metal stenting in the DEB group; c DES restenosis; d BMS restenosis. Trial Acronyms: DEB-AMI: Drug-eluting Balloon in Acute Myocardial Infarction; DEBIUT: Drug-eluting Balloon in Bifurcations Trial; PERfECT: Prospective, Randomised Trial Evaluating a Paclitaxel-Coated Balloon in Patients Treated with EPC Stents for De Novo Coronary Artery Disease; PEPCAD III: DEBlue Stent versus Cypher Stent in the Treatment of Advanced Coronary Artery Disease; PEPCAD IV DM: Paclitaxel Eluting Balloon Versus Drug Eluting Stent in Native Coronary Artery Stenoses of Diabetic Patients; BELLO: Balloon Elution and Late Loss Optimization; PICCOLETO: Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels; PACCOCATH – ISR I (II) Treatment of in-Stent Restenosis by Paclitaxel Coated PTCA Balloons; PEPCAD DES: Treatment of DES-In-Stent Restenosis With SeQuent® Please Paclitaxel Eluting PTCA Catheter; PEPCAD II: The Paclitaxel-Eluting PTCA-Balloon Catheter in Coronary Artery Disease to Treat In-Stent Restenoses.
The most significant, still unresolved issue with DEB is the limited number of patients enrolled in the randomised trials with this technology. Interventional cardiologists are also eagerly waiting for the answer to two additional questions regarding DEB: are there differences in performance between currently available DEB and what are the reasons for the striking failure of DEB plus BMS considering the enormous success of their combination in the form of DES? Two studies published in the current issue of EuroIntervention contribute to the clarification of these two questions. Bondesson and colleagues retrospectively compared two paclitaxel-eluting balloons in patients undergoing PCI and included in the Swedish coronary and angioplasty register (SCAAR/Swedeheart)27. Of a total of 1,136 patients, 919 patients were treated with a balloon eluting 3 μg/mm2 of paclitaxel from a layer of hydrophilic contrast media as excipient (Sequent Please, B. Braun AG, Melsungen, Germany), and 217 patients were treated with a balloon eluting 2 μg/mm2 of paclitaxel directly from its surface without the use of an excipient (Elutax, Aachen Resonance GmbH., Aachen, Germany). DEB with hydrophilic carrier were associated with a lower risk of restenosis (adjusted hazard ratio 0.39 [95% confidence intervals 0.24-0.65]) at an average follow-up of ~11 months. This advantage was observed for both de novo and restenotic lesions. Interestingly, most of failures in patients treated with DEB without excipient occurred <6 months after index procedure, suggesting poor drug retention in the vessel wall. As a closing remark, the authors suggest the lack of a “class-effect” for DEB and the urgent need for head-to-head, randomised comparisons of different DEB platforms. These findings provide additional support to the statement included in the European guidelines on myocardial revascularisation that “one cannot assume a class effect for all drug-eluting balloons”26.
In the second study, Fischer and colleagues present the data from a subgroup of 55 patients who performed intravascular ultrasounds (IVUS) at nine-month follow up in the DEBlue Stent versus Cypher Stent in the Treatment of Advanced Coronary Artery Disease III (PEPCAD III) trial28. The original trial randomly assigned 637 patients to receive DEB-BMS (Coroflex DEBlue, B. Braun AG, Melsungen Germany) versus sirolimus-eluting stents (SES, Cypher, Cordis, Miami Lakes, FL, USA) for de novo lesions. Current findings show that despite the similar stent expansion (symmetric expansion index 0.90±0.02 versus 0.89±0.04 for DEB-BMS versus SES, respectively, p value not significant) and strut apposition between DEB-BMS and SES, risk of restenosis was higher in the DEB-BMS group, as shown in the primary trial29. The main objective of this IVUS sub-study was, however, to assess whether a possible “geographical mismatch” (BMS deployment in segment of the vessel not pre-treated with DEB) underlies the lower antirestenotic efficacy of DEB-BMS. The authors excluded this as the failure mechanism of the DEB-BMS strategy. However, they only did an indirect assessment of “geographical mismatch” based on the lack of excess neointima at the stent margins in the DEB-BMS group. A more precise assessment would have required acute intravascular imaging during the index procedure and probably more sensitive imaging technology such as optical coherence tomography30.
As a matter of fact, previous randomised trials failed to definitely address the merits and pitfalls associated with the concurrent use of DEB and BMS in de novo lesions, whilst the utility of DEB in case of ISR has been better scientifically proven. The limited number of patients enrolled in these trials did not allow identifying whether the failure of DEB-BMS was due to a strategy of “balloon first” or “stent first” angioplasty, or to stent implantation per se10,14. At a mechanistic level it may be argued that the time-limited antirestenotic effect of DEB cannot be sufficient to antagonise the chronic proliferative stimulus generated by BMS18. Moreover, whether the presence of BMS may act as a barrier that prevents the transfer of the drug into the vessel wall still remains a matter of some debate31.
As DEB technology rapidly evolves, possible ameliorations are largely desirable16. The use of new antiproliferative drugs, as well as the adoption of new excipients that enhance drug transfer and retention in the vessel wall, need further investigation. Similarly it warrants further study whether other endovascular treatment approaches, such as cutting balloons, might act in synergism with DEB by augmenting drug penetration at the PCI site. Finally, new imaging technologies may better meet our need for a more comprehensive analysis of DEB interactions with the vessel wall. Most importantly we need trials that assess the role of DEB in a well-defined patient population with a sufficiently large sample size. A total of 18 trials have been registered at the clinicaltrial.gov database; some of these are expected to be presented or published in the next few months including:
– the Efficacy Study of Paclitaxel-eluting Balloon -Stent versus Plain Angioplasty for Drug-eluting Stent Restenosis- ISAR DESIRE 3, NCT00987324;
– the Restenosis Intra-stent of Drug-eluting Stents: Paclitaxel-eluting Balloon versus Everolimus-eluting Stent - RIBS IV, NCT01239940; and
– the Treatment of Coronary Artery Disease With Bare Metal Stent Followed by Paclitaxel-Coated Balloon Catheter Versus Paclitaxel-Eluting Stent - SEQUENT 1000, NCT01166711.
They will certainly contribute with valuable information in establishing the real role of DEB as a PCI option.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References

