
Abstract
Four decades after its introduction into clinical practice, coronary balloon angioplasty is still used during most coronary interventions. Conventional balloon angioplasty is frequently used to predilate complex or severe lesions and remains of major value to optimise the results of stent implantation. Plain balloon angioplasty is still used alone in some anatomic scenarios where stent implantation is not desirable (very small vessels or diffuse lesions, large resistant thrombus burden, side branches of bifurcations). However, this technique is hampered by a relatively high restenosis risk. Recently, drug-coated balloons (DCB) have been shown to provide an attractive new tool for the “leave nothing behind” strategy. Many studies have demonstrated that DCB are indeed safe and effective. Evidence of the value of DCB in patients with ISR is overwhelming. DCB are attractive for selected de novo coronary lesions (small vessels, diffuse disease, side branches). DCB have also gained major evidence supporting their clinical efficacy in the peripheral arterial territory. Further studies are required to elucidate the relative value of DCB compared with alternative strategies (namely new-generation drug-eluting stents) in different clinical and anatomic scenarios.
Abbreviations
BMS: bare metal stents
BTK: below the knee
DAPT: dual antiplatelet therapy
DCB: drug-coated balloons
DES: drug-eluting stents
EES: everolimus-eluting stents
ISR: in-stent restenosis
LLL: late lumen loss
MACE: major adverse cardiac events
PCI: percutaneous coronary intervention
PTA: percutaneous transluminal angioplasty
PTCA: percutaneous transluminal coronary angioplasty
SFA: superficial femoral artery
SVD: small vessel disease
TLR: target lesion revascularisation
BALLOON ANGIOPLASTY
Historical background
Forty years ago, Andreas Grüntzig performed the first percutaneous balloon coronary angioplasty procedure. On 16 September 1977, for the first time ever, he dilated a coronary stenosis located in the proximal left anterior descending coronary artery in a 38-year-old conscious patient in the University Hospital of Zurich (Switzerland)1-5. A pioneer balloon catheter, including a soft wire stub fixed to the tip that could be pre-shaped manually (Grüntzig Dilaca DG 20-30, manufactured by Schneider), was used. This balloon catheter was not steerable but had an additional distal hole to allow pressure measurements. The procedure was performed with documentation of the aortic pressure, from the tip of the guiding catheter, and simultaneous recording of the pressure distal to the lesion, from the tip of the balloon catheter. At the time, pressure recordings were considered critical as good angiographic pictures could only be obtained a posteriori when the 35 mm cine film was processed. The intervention was organised to be able to infuse the distal coronary vessel through the distal lumen of the balloon catheter with blood from the contralateral femoral artery. However, the procedure went very smoothly and this bail-out strategy was eventually not required1-5. This intervention was a critical milestone heralding the progress we have seen in the field during the last four decades1-7.
Years before, Andreas Grüntzig, while a young radiologist doing his fellowship in Germany, learnt the Dotter technique to treat peripheral arterial disease1-5. Charles Dotter, a vascular radiologist, described his technique following the unintentional recanalisation with a catheter of an occluded iliac artery in a patient investigated for a renal artery stenosis. This technique, consisting in the use of sequential intraluminal dilators, opened new avenues in the treatment of occluded vessels. Of interest, Charles Dotter also first envisaged the potential use of balloon catheters and endoluminal stents (at the time called endovascular splints) to improve results of vascular revascularisation, although he was unable to develop these technologies further1-5. On the other hand, Andreas Grüntzig realised that the latex devices previously tried by Dotter (Fogarty embolectomy balloons) were unable to dilate vascular stenosis caused by atherosclerosis. He found, however, that the less elastic polyvinyl chloride plastics provided adequate force in his initial homemade balloons. His early experiments were performed in his own home kitchen, where balloons were customised for each patient, opening the era of “personalised medicine”1-5. In 1973, a single-lumen balloon catheter was tested in animals and, in 1974, this balloon was used to dilate the superficial femoral artery of a patient1-5. Grüntzig subsequently developed double-lumen polyvinyl balloon catheters to enable pressure monitoring and distal contrast injections. These balloons were able to provide predefined constant dilating pressures. In 1975, the first double-lumen balloon catheter was used for a femoral angioplasty. Then, these catheters evolved and became small enough to be used in the coronary arteries. Accordingly, Grüntzig was able to start experimental coronary studies in animals and also in cadavers. In May 1977, in San Francisco, with the help of his friend Richard Myler, he used these balloons in living patients during coronary surgery1-5. Lesions on the proximal left anterior descending coronary artery were retrogradely dilated in the operating room prior to performing the anastomosis of the bypass graft. These surgical procedures were conducted immediately before the first successful percutaneous coronary procedure back in Zurich. Interestingly, that very first patient was initially scheduled for bypass surgery, but Ake Senning, chief of surgery at the time, not only allowed the procedure to be organised but also kindly provided surgical stand-by1-5. To gain perspective, this first coronary intervention was performed a decade after Rene Favaloro introduced aortocoronary bypass surgery.
In 1980, Andreas Grüntzig joined the Emory University in Atlanta to develop his technique further. The intervention was initially received by the scientific community with major scepticism and concerns regarding the potential risks of vessel rupture, major dissection or distal coronary embolisation. Andreas Grüntzig skilfully recognised the risk of acute occlusion and late restenosis as the main caveats of the procedure that he insisted be used with major caution for the clinical benefit of well-selected patients6,7. He used to remark that his main legacy to medicine would be to have been working on the human heart in a conscious patient. Andreas Grüntzig died on 27 October 1985, at the age of 46, in an accidental crash while piloting his twin-engine plane returning to Atlanta1-5.
The early stages and development of balloon angioplasty
The procedure was initially named “percutaneous transluminal dilatation” or “percutaneous transluminal recanalisation” but evolved to “percutaneous transluminal angioplasty” (PTA) in peripheral vessels or “percutaneous transluminal coronary angioplasty” (PTCA) in the coronary territory1-5. The term “percutaneous coronary intervention” (PCI) is nowadays preferred as stents are implanted in the vast majority of cases. In less than a decade, balloon catheters (initially bulky and with low burst pressures) improved significantly, resulting in smaller profiles and the possibility to attain high inflation pressures. At the same time, clinical experience grew dramatically and procedures in more complex patients were attempted. Patients with acute coronary syndromes, including acute myocardial infarction, and patients with multivessel disease were treated. Ad hoc procedures (interventions during the same diagnostic procedure) were frequently used. Multivessel balloon angioplasty was widely embraced, initially performed with staging of the procedures and, thereafter, during the same setting1-5.
From a technical standpoint, the use of steerable guidewires increased the rates of successful crossing of the lesion from 70% to more than 90%8. Fixed wires evolved to “over-the-wire” systems that required a second lumen for the long wire running the length of the catheter. Subsequently, the monorail technology (rapid exchange system) incorporated a second lumen but only confined to the distal 10-25 cm of the catheter8. This facilitated the performance of the technique by a single operator. Polyvinyl chloride balloons were relatively compliant but lost their profile after the first inflation (developing bulky wings). Polyethylene terephthalate balloons permitted the maintenance of a relatively constant balloon size over a wide range of pressures8. Smaller profile balloons with different coatings or lubricants were developed to reduce friction caused by the system and to facilitate crossing of the lesion. Typically, catheter shafts made of polyethylene improved trackability, whereas stiffer polyvinyl chloride shafts provided better pushability8. Some very tiny balloons incorporating fixed distal wires were also developed for selected cases but never gained widespread adoption. These procedures were facilitated by the use of guiding catheters with better passive support or with soft tips, allowing deep coronary intubation to provide active support8.
The evolution of the technique in the USA was nicely described by the National Heart, Lung, Blood Institute Dynamic Registry reports9-11. These reports illustrated the evolution of the technique with increasing success rates. Major attention was paid to the role of clinical (age, gender, clinical presentation [stable vs. unstable angina], diabetes, renal disease), anatomical (lesion severity and length, vessel size, calcium, tortuosity, bifurcation, total occlusion, left ventricular ejection fraction) and technical factors (balloon-to-artery ratio, pressure, inflation time, residual stenosis, residual dissections) in relation to both the acute and the long-term procedural results9-11. This information was critical to define better the risk of acute complications and to inform revascularisation decisions. Major attention was paid to identifying factors predicting restenosis. Adverse angiographic lesion characteristics (that were lumped together in the A, B1, B2 and C classification) identified predictors of complications but also of late restenosis. Most of these variables still play a major prognostic role in the current era of coronary stenting. As residual dissections were systematically detected once the quality of the angiographic equipment improved, a classification scheme (NHLBI dissections A to F) was devised to define these dissections. Type A-B dissections were considered not only benign but, interestingly enough, were associated with better long-term clinical outcome, resulting in a reduced angiographic restenosis rate. However, more advanced dissection grades were associated with a high risk of acute vessel closure9-11 (Figure 1, Figure 2).
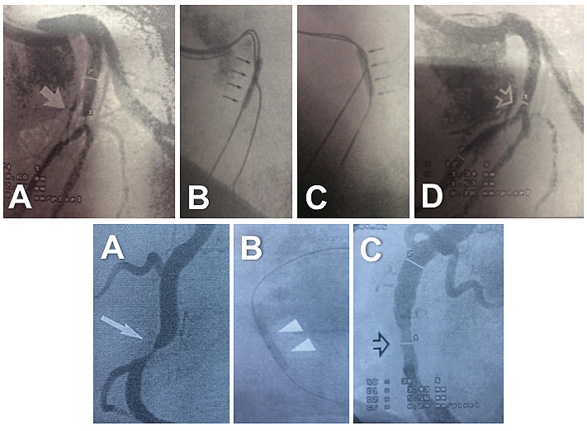
Figure 1. Balloon angioplasty procedures performed in the late 1980s. Top: A) treatment of a severe lesion (arrow) located in a bifurcation. An 8 Fr guiding catheter was used. Notice early digital technology with edge enhancement. Sequential balloon angioplasty first to the left anterior descending coronary artery (B), and then to the diagonal branch (C) was performed. D) Final angiographic result (open arrow). Bottom: A) treatment of a severe lesion in the right coronary artery (arrow). B) Balloon dilation. Notice the completely radiopaque wire. C) Final result (open arrow) showing moderate residual stenosis without a clear dissection.
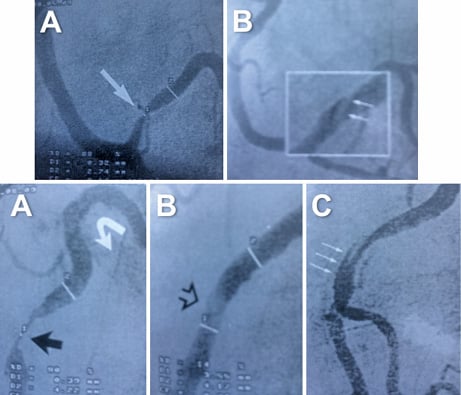
Figure 2. Balloon angioplasty procedures performed in the 1980s. Top: A) severe lesion in a large posterolateral branch. B) After balloon angioplasty, a type B coronary dissection was recognised (magnification). Bottom: balloon angioplasty of a severe lesion (black arrow) located in a tortuous (shepherd’s crook morphology) right coronary artery (A). After dilation, a clear lumen gain was obtained with significant haziness at the dilated segment (B). A type C dissection was revealed in an orthogonal view (C). Both patients were left with untreated residual dissections and had good clinical outcomes.
Insights on the pathophysiological mechanisms of success and failure
Balloon angioplasty produces a major barotrauma with deep vessel injury. Plaque compression is not a significant mechanism of lumen dilation. Actually, the main mechanisms of lumen enlargement include plaque rupture with vessel dissection and overstretching of the entire vessel wall12,13. Accordingly, the main limitations of this technique, namely acute vessel occlusion and restenosis, were also closely related to its mechanism of action. Acute vessel closure may occur in up to 5% of patients, and is a result of an occlusive dissection with or without thrombus. In the early days, vessel closure was a frequent cause of myocardial infarction and, at the time, it was considered an indication for urgent coronary surgery6,7. Restenosis (typically defined as a >50% lumen diameter stenosis) tends to occur within the first six months after the procedure as a result of neointimal proliferation and late elastic vessel remodelling12,13. Clinical restenosis rates after balloon angioplasty are 20-25%, whereas angiographic restenosis rates (on systematic angiographic surveillance) may range from 30 to 50%.
The stretching process enlarging the lumen causes plaque fracture due to inelastic components of the atheroma12,13. Acute denudation of the endothelium is a consistent feature. This promotes marked acute platelet adhesion and aggregation depending on the degree of vascular injury. Subsequently, thrombus formation, smooth muscle cell proliferation and a new fibrocellular proliferative process occur. Restenosis proved to be a multifactorial process14. Prevention efforts by inhibiting platelet accumulation, thrombus formation and smooth muscle cell proliferation, by either drug or mechanical means, were attempted for decades with very limited success12,13.
Initial evidence suggested that intimal hyperplasia was the main mechanism of restenosis after balloon dilation12,13. Lesion characteristics and regional flow dynamics were considered important local biologic determinants of this process. A low wall shear stress was suggested to promote intimal hyperplasia and cause structural changes on the vessel. Intimal hyperplasia proved to be a complex phenomenon involving platelets, growth factors, endothelial cells, smooth muscle cells and wall shear stress. Platelets contribute with platelet-derived growth factors and organised thrombus. Growth factors initiate smooth muscle cell proliferation12,13. Intracoronary imaging demonstrated that, in most patients with angiographically successful balloon angioplasty, residual plaque area was significant (>50%)15. Minimal luminal areas and residual plaque area emerged as predictors of restenosis, whereas dissections had a protective effect. Imaging studies demonstrated that the main mechanism of restenosis after balloon angioplasty was negative vessel remodelling rather than neointimal hyperplasia15.
In the late 1980s several devices were developed in an attempt to prevent restenosis10,11. Although many of these (rotational or directional atherectomy, excimer laser) proved to ablate atherosclerotic plaque efficiently, none of them was able to reduce the restenosis rate consistently10,11. Coronary stents, however, prevailed once the adequate antiplatelet regimen was able to abolish the risk of early thrombosis initially seen when these devices were used with aggressive anticoagulation regimens. Stents provided stable acute scaffolding of the vessels (reducing the risk of acute closure) and, due to their larger acute lumen gain, nicely accommodated the increased neointimal proliferation, eventually providing significantly larger lumens at follow-up.
Current use of balloon coronary angioplasty
Currently, balloon angioplasty is mainly considered a complement to coronary stenting. Balloon angioplasty is frequently used to prepare the lesion before stenting. Except in the case of direct stenting, used in favourable lesions, balloon predilation remains customary in patients with severe lesions. Stent implantation without balloon predilation may lead to stents being deployed in undilatable lesions. This is a challenging scenario because rotational atherectomy can no longer be used to ablate calcium once the vessel has been stented. Severely underexpanded stents represent a major risk factor for stent thrombosis and restenosis.
Likewise, high-pressure balloon inflation is frequently required after stenting (post-dilation) to optimise final results. Recently, this recommendation has also been emphasised after implantation of bioresorbable vascular scaffolds. Ideally, short non-compliant high-pressure balloons should be used during post-dilation. Guidance with intracoronary imaging may provide unique diagnostic insight. Currently, novel very high-pressure balloons may be selected in cases of resistant coronary lesions.
However, currently, a balloon strategy alone is rarely indicated in patients undergoing coronary interventions. To prevent restenosis, the bigger is better adage also works for balloon angioplasty. However, the use of balloon-to-artery ratios >1:1 may result in deeper dissection that enhances the risk of total vessel closure. Accordingly, during balloon angioplasty, a prudent balance between lumen gain and residual dissection should be contemplated when a balloon strategy alone is pursued. Most residual dissections heal spontaneously during follow-up16. In addition, inflation time may be important. Relatively long inflation times may be required to stabilise occlusive dissections after balloon angioplasty when stenting is not indicated.
The first scenario for balloon angioplasty alone is when stents cannot be advanced to the target lesion. With currently available technology, however, this is very rare, but can still occur in very distal lesions in vessels showing tortuosity or severe calcification. Another setting is in small and diffusely diseased vessels where the use of multiple, long stents may not be appealing. Likewise, the use of stents is not very attractive in lesions with very large (resistant) residual thrombus burden17. In this scenario, the operator may accept a suboptimal initial result with balloon dilation alone (ideally preceded by thrombectomy). This conservative strategy might prevent complications such as the no-reflow phenomenon or late stent malapposition, once thrombus resolution is completed. Stent implantation may be deferred a few days hoping for thrombus resolution under aggressive antithrombotic therapy.
Another reason to avoid stent implantation is in lesions located in a side branch of a bifurcation lesion18. In this scenario, a provisional stenting strategy is usually recommended. This includes main vessel stenting, with a proximal optimisation technique, and provisional side branch stenting. Balloon dilation of the side branch is required when the branch presents significant proximal disease or when its origin worsens after main vessel stenting15.
In de novo lesions where coronary stenting is not indicated for different reasons, drug-coated balloons (DCB) should be considered rather than plain balloon angioplasty (Figure 3).
Figure 3. Examples of patients treated with balloon angioplasty alone. Top: A) very long diffuse lesion (yellow arrows) in the left circumflex coronary artery that was a very small vessel in its distal segment. B) & C) After sequential predilation with conventional balloons, the complete disease segment was treated with DCB (trying to avoid the geographic miss phenomenon). D) Satisfactory final results were obtained along the vessel although a type B dissection was noticed at its proximal segment. Bottom: A) Severe lesion in a diagonal branch (arrow) that bifurcated into two branches. B) After adequate predilation a DCB was used. C) Excellent final angiographic results.
DRUG-COATED BALLOONS
History and mechanism of action
Neointimal hyperplasia is seen as a slow process suggesting the need for sustained drug release for restenosis prevention19. The key requirement for local drug delivery independent from a stent would be a rapid drug uptake by the tissue and drug persistence in the vessel wall to compensate for a short contact time20. In the late 1990s, Christian Herdeg and colleagues investigated the local application of paclitaxel using “double-balloon” or “porous balloon” catheters in the animal model21. Creel et al22 reported that uptake of paclitaxel into the arterial wall was twentyfold increased over heparin after 15 minutes of exposure. Although a process taking 15 min is too long by far to be applicable during PTCA, the experiment indicated that lipophilic drugs are rapidly taken up by the tissue. Furthermore, it could be demonstrated that competitive binding, e.g., by albumin and other plasma proteins, results in diminished paclitaxel accumulation in the arterial wall23.
In 1999, Ulrich Speck and Bruno Scheller started their joint research projects on new concepts in local drug delivery. The reason for selecting taxane compounds (protaxel, paclitaxel) was the group’s access to them and the more complex patent situation with rapamycin analogues at that time24. They made the surprising observation that bolus injection of contrast media through coronary arteries allowed taxane uptake to the vascular wall sufficient to inhibit restenosis in the porcine model25,26. However, since vasculature is multi-branched, dose control was challenging27. Therefore, they were looking for a more lesion- than vessel-specific way of intravascular drug delivery. A variety of different coatings on balloon catheters was investigated24 (Figure 4). A balloon coating using the contrast medium iopromide as excipient showed a dose-dependent reduction of neointimal formation in the porcine coronary model at one-month follow-up28. After presenting these findings, the research group was exposed to almost complete refusal of their DCB concept. Neither physicians nor medical device companies could believe that the drug release from a balloon catheter during the short-lasting inflation time might be as efficacious as sustained release from permanently implanted stents. At that time, funding for further research was complicated. The first patent applications were filed with the support of Charité University Hospital, Berlin, Germany. At the end of 2003 a small first-in-man trial in ISR was initiated (PACCOCATH ISR)29.

Figure 4. Prototype drug-coated balloon catheters (ca. 2001) and clinical samples for the first-in-man trial (2003).
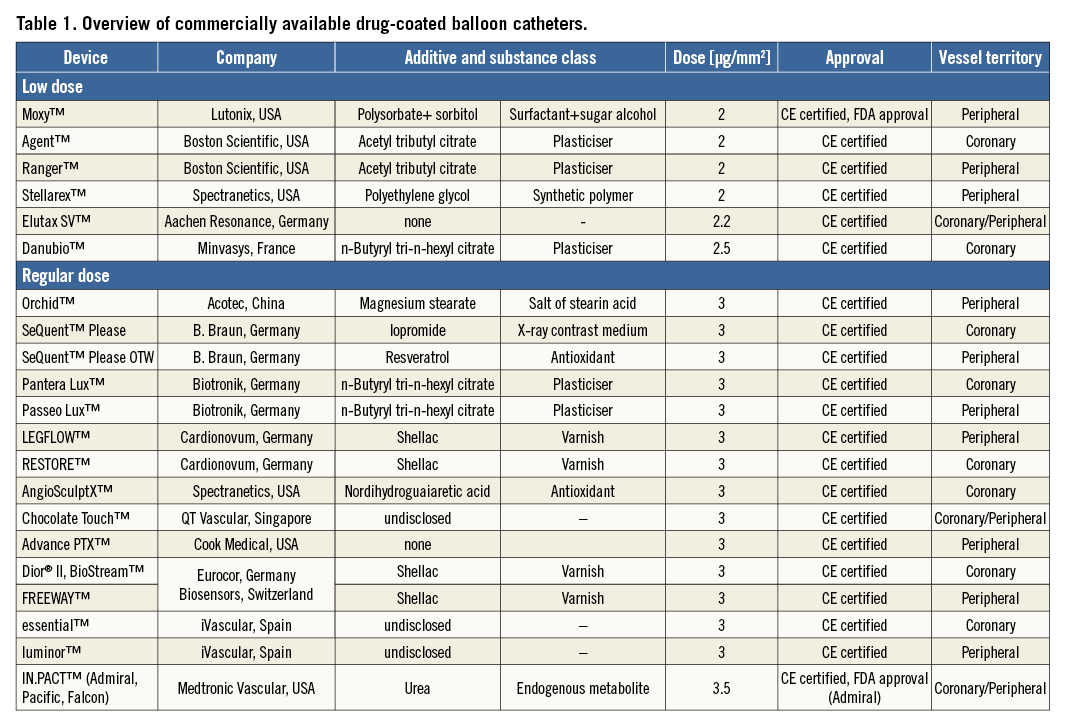
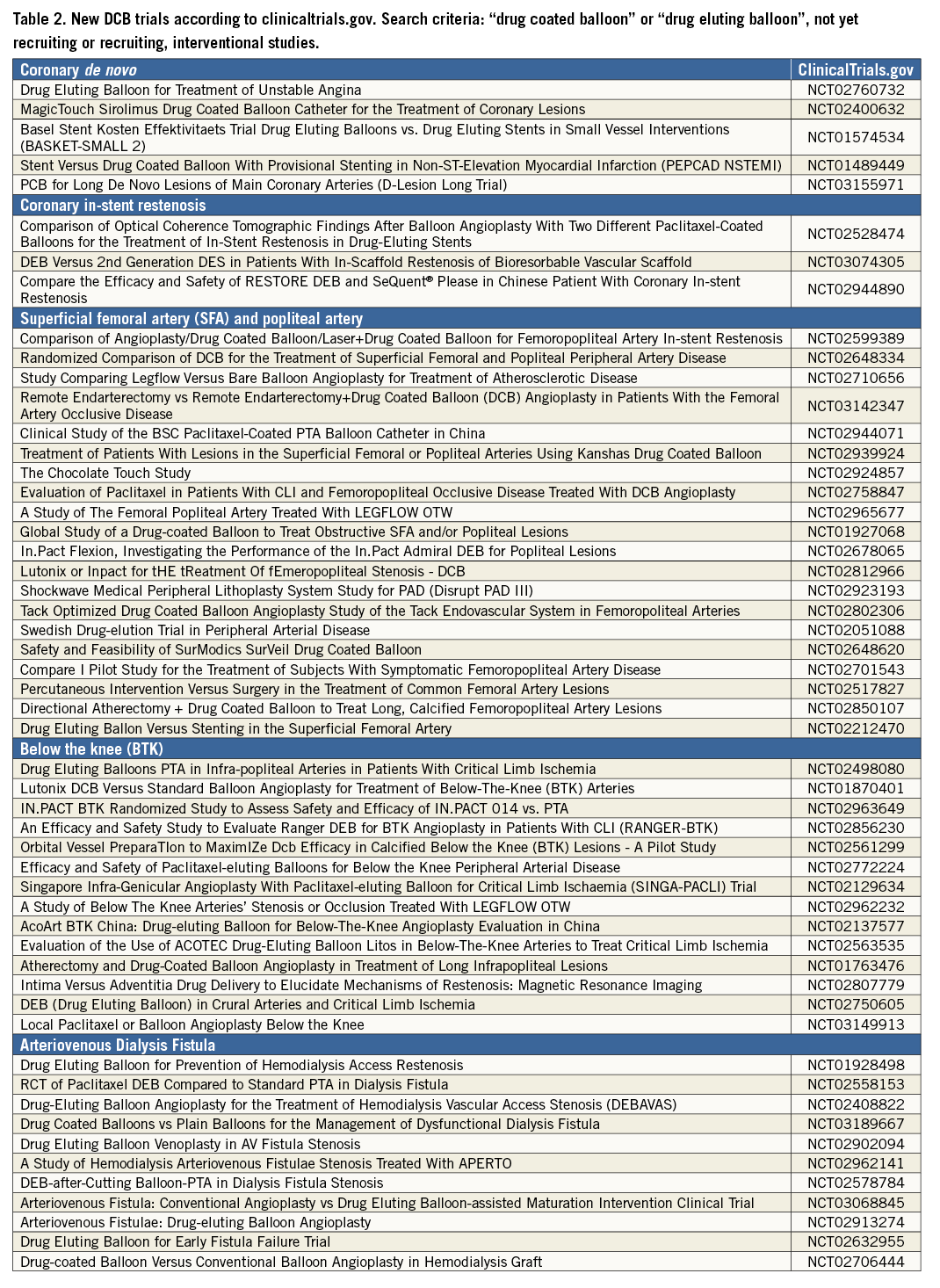
After the initial studies had been published, several manufacturers started developing DCB. Meanwhile, in Europe, a variety of different devices was available for coronary and peripheral use, and two peripheral DCB gained FDA approval in 201530,31. Currently, paclitaxel is the drug of choice with the typical dosage being between 2 and 3.5 μg/mm² of balloon surface. The critical factors enabling successful drug transfer are the coating formulation and coating procedure, resulting in different pharmacokinetic profiles20 and different clinical outcomes32. In this respect, the interaction between drug doses, formulations, release kinetics and lesions plays a fundamental role in the vascular response after DCB therapy, without signs of a “class effect” among platforms. Table 1 summarises currently available DCB, whereas Table 2 lists new, ongoing or just approved studies or clinical trials in different lesions and territories.
Clinical data
CORONARY ARTERIES
IN-STENT RESTENOSIS (ISR)
Despite having positive animal data from the porcine coronary stent model28, there was some uncertainty about the best scenario for a first-in-man study. Ulrich Speck and Bruno Scheller discussed the possibility of limited efficacy versus potential safety issues as seen with brachytherapy33 or early drug-eluting stent (DES) concepts34. They decided to select patients with bare metal stent (BMS) ISR, a patient population with a high risk for restenosis and lack of good treatment options in 2003, and the safety net provided by several layers of neointimal cells covering a previously implanted stent. The PACCOCATH In-Stent Restenosis I trial was a German, controlled, randomised, first-in-human, multicentre study with blinded angiographic evaluation comparing the efficacy and tolerance of the Paccocath™ (Bavaria Medical Technology, Munich, Germany) DCB with conventional uncoated catheters for the treatment of BMS ISR. Compared to patients treated with an uncoated balloon, patients in the Paccocath balloon group had significantly better angiographic results (in-segment late luminal loss 0.74±0.86 versus 0.03±0.48 mm; p=0.002) and concomitant 12-month clinical outcomes29. A second trial added 56 patients with similar baseline clinical and angiographic data. The most surprising finding was that the beneficial effects of the Paccocath DCB were maintained for up to five years after the intervention35. Importantly, in contrast to DES, combined antiplatelet therapy was continued only for one month followed by treatment with aspirin alone. Later on, the original “Paccocath” coating was improved for coronary use (SeQuent® Please; B. Braun) and studied in different clinical trials29,36-45, leading to a class I level A recommendation for the treatment of coronary ISR in the 2014 European guidelines46.
Current research in ISR treatment is focused on the comparison with current-generation DES, improved vessel preparation before DCB, and long-term outcomes. The implantation of a second stent for the treatment of ISR results in better initial lumen gain. The Spanish RIBS IV trial showed a significant advantage of everolimus DES (EES) vs. DCB for the treatment of DES ISR in angiographic endpoints and target lesion revascularisation (TLR)44. On the other hand, DCB treatment avoids several layers of metal47, reduces the need for prolonged dual antiplatelet therapy, allows repeatability of the procedure48, and could positively influence hard clinical endpoints on longer follow-up49,50. For example, after three years in ISAR-DESIRE 3, the hazard ratio for overall mortality was 0.38 (6.0 vs. 15.3%, p=0.02) and 0.27 for cardiac mortality (p=0.03) in favour of DCB vs. first-generation DES in the treatment of DES ISR. It is important to note that this benefit was not related to reintervention rates49. This finding might be explained by an elevated stent thrombosis risk with sandwich DES51. In the three-year follow-up of the RIBS V trial, safety issues in the treatment of BMS ISR with EES were not observed52.
Lesion preparation before DCB use is mandatory to ensure sufficient initial lumen gain. Since it is hard to achieve stent-like results with balloon angioplasty alone, the German DCB consensus group proposed the term “acceptable result” after lesion preparation. Acceptable results exclude flow-limiting dissections (type A and B are allowed), a reduced TIMI flow, and a diameter stenosis of more than 30%. If the requirements of an acceptable result are fulfilled, “DCB-only” seems to be feasible53 and is associated with a significant reduction of TLR at one year compared to an inappropriate angioplasty result54. Lesion preparation with scoring balloons before DCB may further improve angiographic outcomes55. Drug-coated scoring balloons could improve lumen gain and facilitate drug transfer56.
CORONARY DE NOVO DISEASE
In clinical routine, DCB use is more frequent in coronary de novo lesions than in ISR, despite the fact that there is no guideline recommendation and large randomised clinical trials are not yet available. This contradiction may be attributable to the initial misconception of creating a new type of “polymer-free DES” when combining DCB with BMS which was inferior to limus DES and, at best, similar to first-generation paclitaxel DES57-59. Patients undergoing standalone DCB treatment in coronary small vessel disease (SVD) showed superior long-term outcomes compared to the combination of DCB and BMS60.
Careful lesion preparation is the first and most important step in the DCB-only strategy to ensure sufficient initial lumen gain and identify lesions at risk for acute vessel closure53. In case of an acceptable result after lesion preparation, the procedure ends with a 30-second DCB inflation at nominal pressure. In case of a major dissection (type C or higher), a residual stenosis of >30%, or reduced flow, the implantation of a limus DES is recommended (Figure 5). In different trials, the need for stent implantation was in the range of 20 to 30% after treatment according to the DCB-only concept39,61-63, comparing well to an optimal provisional stent rate of 20 to 40%64. MACE rates in large registries at nine months were 4.7% in SVD63 and 2.6% in larger coronary arteries39. It is important to note that there was no safety signal in terms of early vessel closure, which may be explained by excluding lesions at risk depending on the result of lesion preparation. Lesions with non-flow-limiting dissections undergoing angioplasty show excellent clinical outcomes65, especially with DCB treatment66. Furthermore, there is no reason to deny a similar beneficial impact of DAPT in angioplasty as was the case in stenting67-69. Local balloon-based drug delivery addresses restenosis and allows luminal gain within four to six months after treatment70,71. Serial intravascular ultrasound studies demonstrated a paclitaxel-induced increase in lumen area and vessel area71, imitating the Glagov effect seen in early atherosclerosis72. Such a lumen enlargement is key for leaving the lesion with an acceptable instead of a stent-like result.
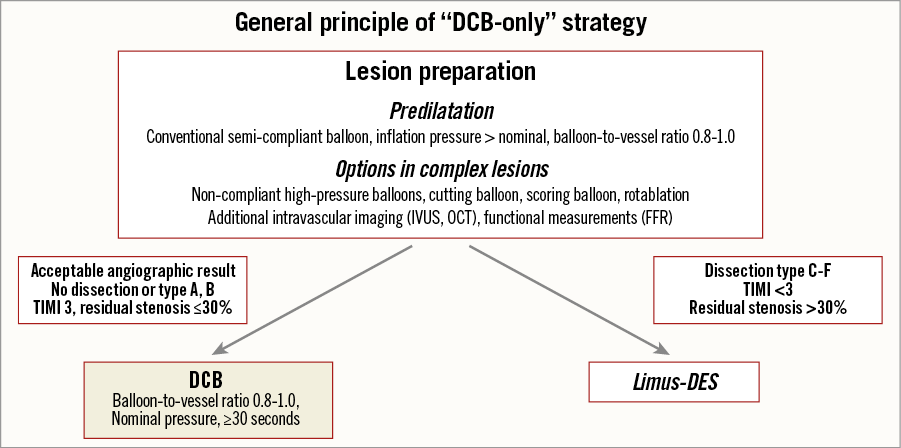
Figure 5. General principle of DCB-only strategy. Adapted from53,104. Original figure was published in EuroIntervention, 201153.
Treatment of coronary SVD was investigated in the BELLO trial comparing DCB and first-generation DES in 182 patients. At six months, late lumen loss (LLL) was significantly lower in the DCB arm (0.08 vs. 0.29 mm, p=0.001)62. Whether LLL is a good measure when comparing stent-based with balloon-based treatments has been the subject of discussion73. Nevertheless, after three years, a significant difference in major adverse events was reported in favour of DCB (14.4% vs. 30.4%, p=0.015), which was driven by death and myocardial infarction74. A recently published propensity score-matched comparison in SVD indicated similar clinical outcomes of DCB when compared with EES (MACE rate at one year 12.2% with DCB and 15.4% with EES)75. Lesion preparation before DCB in SVD with a scoring balloon (NSE ALPHA; Nipro Corporation, Osaka, Japan), led to a significantly higher minimal lumen diameter post intervention and a significant reduction of bail-out stenting or a residual stenosis of more than 50%76. A small randomised study comparing scoring balloon followed by DCB versus EES showed no difference in angiographic outcomes at six months. Target lesion reintervention was 0 after scoring balloon+DCB vs. 6% with EES (p=NS)77. Patient inclusion in the large-scale randomised BASKET-SMALL 2 trial comparing EES with DCB in SVD was completed in February 2017 (NCT01574534); the primary clinical endpoint at one year will be presented in 2018.
In bifurcation treatment, DCB+BMS in the main branch of bifurcations did not reveal any benefits compared to limus DES59. The role of DCB in bifurcations may be a puristic approach with DCB-only in the main and side branch78 or a limus DES in the main branch and DCB in the side branch. Two randomised studies showed a significant reduction of LLL and binary restenosis in the side branch by DCB (primary endpoint LLL in PEPCAD-BIF 0.13 mm in the DCB vs. 0.51 mm in the conventional balloon group, p=0.013)79,80.
PERIPHERAL ARTERIES
SUPERFICIAL FEMORAL ARTERY
In parallel to initiating the coronary first-in-man studies, two first-in-man trials in the superficial femoral artery (SFA) were proposed. Key drivers were Gunnar Tepe together with Thomas Zeller (THUNDER)27 and Jens Ricke (FemPac)81. Both trials investigated the original Paccocath coating. In the THUNDER trial, 154 patients were treated with conventional PTA, Paccocath DCB, or a third arm with paclitaxel dissolved in the contrast medium. After six months, treatment with Paccocath DCB was associated with significant reductions in LLL compared to patients in the uncoated balloon group or patients treated with paclitaxel dissolved in the contrast medium27. Importantly, the rate of TLR remained significantly lower in the Paccocath group up to five years of follow-up82. The FemPac trial confirmed the superiority of Paccocath DCB over PTA in the SFA81.
Several coatings different from the original Paccocath generated a wider data set in peripheral artery disease. A coating formulation using urea as excipient and paclitaxel at a dose of 3.5 μg/mm² was developed in 2008 (IN.PACT; Medtronic, Dublin, Ireland)83. In the PACIFIER trial, this coating showed a slightly negative late lumen loss of -0.01 mm versus 0.65 mm for PTA (p=0.001)84. In the IN.PACT SFA pivotal trial, 331 patients with claudication or rest pain due to SFA lesions were enrolled. At one year, the DCB group was superior to PTA in terms of primary patency (82% vs. 54%), clinically driven TLR (2.4% vs. 21%), primary sustained clinical improvement (85% vs. 69%), primary safety endpoint (96% vs. 77%), and MACE (6% vs. 24%)85. At two86 and three years a sustained benefit with regard to primary patency and clinically driven TLR was reported. Other randomised studies confirmed those results including treatment of restenotic lesions87.
The Orchid® DCB (Acotec Scientific, Beijing, China) is coated with 3 μg/mm² paclitaxel and magnesium stearate. In the AcoArt trial, relatively long lesions were treated (about 15 cm on average). Late lumen loss was 0.05 mm with coated balloons versus 1.15 mm with uncoated balloons (p<0.001). At one year, the rates of TLR were 7.2% and 39.6%, respectively (p<0.001)88. Furthermore, positive results have been published from randomised trials investigating DCB coated with 3 μg/mm² paclitaxel and using n-Butyryl tri-n-hexyl citrate (Passeo Lux; Biotronik, Berlin, Germany)89 or resveratrol (SeQuent Please OTW; B. Braun)90 as excipients.
The LEVANT I trial investigated a DCB coated with 2 μg/mm² paclitaxel using polysorbate and sorbitol as excipients (Moxy; Lutonix, Maple Grove, MN, USA). Late lumen loss at six months was significantly reduced (0.46 mm vs. 1.09 mm) by Moxy whereas TLR did not differ significantly between the two treatments (13% vs. 22%)91. In the LEVANT II pivotal trial including 476 patients, primary patency at 12 months was 65.2% for the DCB and 52.6% for control angioplasty (p=0.015). Freedom from clinically driven TLR was similar in both groups (38.0 vs. 37.5%)92.
The ILLUMENATE FIH trial reported 50 patients treated with a paclitaxel-coated DCB with 2 μg/mm2 and polyethylene glycol (Stellarex™; Spectranetics, Colorado Springs, CO, USA) after predilatation; the study population without predilatation was not reported. Late lumen loss at six months was 0.54 mm93. In the ILLUMENATE EU RCT, primary patency at one year was 83.9% vs. 60.6% in the case of PTA. In contrast to other randomised trials, patients with provisional stenting were excluded from primary analysis; in this DCB subpopulation primary patency at one year was only 78.8%94.
Clinically established excipient-based paclitaxel coatings cause a dose-dependent suppression of neointimal formation with a maximal effect at about 3 to 4 μg/mm228,83,95. A recent meta-analysis reported a significant reduction of restenosis (RR 2.1, 95% CI: 1.2 to 3.4, p<0.001) and TLR (RR 2.5, 95% CI: 1.9 to 3.8, p<0.001) by DCB with a regular dose of 3.0-3.5 μg/mm2 compared to low-dose DCB with 2 μg/mm2 32.
BELOW THE KNEE (BTK)
Restenosis rates in infratibial artery disease range from 42% at 12 months for short lesions to 69% at three months for a lesion length of >18 cm. Initial non-randomised series and one randomised study indicated favourable outcomes with the IN.PACT Amphirion DCB in this challenging scenario96,97. However, the IN.PACT DEEP trial using the same device could not confirm these findings. Compared to PTA, no biological effect was seen in terms of LLL or TLR. Furthermore, there was a statistical trend in terms of major amputations at one year (8.8 vs. 3.6%; p=0.08)98. Several factors have been discussed as reasons for these findings, such as different protocols for wound care in higher Rutherford classes, device-specific issues such as drug loss on the way to the lesion, or simply (by chance) the best ever reported PTA outcomes in such a patient population. Also, another device (Passeo Lux) did not show a significant benefit in this indication99. Since conflicting data exist on the impact of DCB below the knee, the results of ongoing trials focusing on patient- (suboptimal retention at follow-up) and trial-specific (no dedicated wound-care units in most trials) aspects including improved devices and protocols for wound care should be awaited.
Future and unmet needs
In the last few years, DCB have become an established therapeutic treatment modality in coronary ISR46 and SFA disease30, with the prospect of becoming standard of care in these indications. However, there is no class effect for DCB, explaining in part the heterogeneous clinical data set available20,32. Another misunderstanding of the technology is related to coronary use and the combination with bare metal stents. The strength of DCB is the concept of leaving nothing behind as opposed to permanent implants but not their combination with stents53. Uncertainty remains in coronary de novo disease due to the lack of published large-scale randomised trials and in BTK due to conflicting data.
In peripheral artery disease, dissections seem to be helpful for DCB treatment100. However, severe calcification limits drug transfer and clinical outcomes. Future trials will address the impact of plaque modification or removal prior to DCB or combining DCB with self-expanding stents or spot-stenting. In coronary application, quality of lesion preparation before DCB determines the clinical outcome54. Special balloons such as scoring or constraint balloons help to improve the angioplasty result and potentially reduce the occurrence of flow-limiting dissections76. Drug coating of such devices could potentially improve drug transfer and simplify the procedure56,95,101. Modifications of the balloon surface may facilitate drug persistence in blood and transfer in case of contact with the vessel wall.
Interventional cardiologists may prefer drugs different from paclitaxel, such as zotarolimus102 or sirolimus103, due to the historical development in DES technology. However, randomised clinical trials are mandatory to clarify a potential role of rapamycin analogues in balloon-based local drug delivery.
Conclusions
Four decades after its introduction into clinical practice in the coronary territory, balloon angioplasty is here to stay. Conventional balloon angioplasty is frequently used to predilate complex or tight lesions and remains of major value to optimise the results of stent implantation. This technique is still used in certain anatomic scenarios where stent implantation is not desirable (very small vessels or diffuse lesions, large resistant thrombus burden, side branches of bifurcations). More recently, however, DCB have provided a novel avenue to advance in the treatment of coronary lesions with an attractive strategy of “leave nothing behind” combined with local drug delivery. Many experimental and clinical studies have convincingly demonstrated that DCB are safe and effective. Evidence of the value of DCB in patients presenting with ISR is overwhelming. Likewise, DCB appear promising for selected de novo coronary lesions (including, in particular, small vessels, diffuse disease, side branches of bifurcation). DCB have also accumulated major evidence supporting their safety and efficacy in the peripheral arterial territory. Further studies are required to ascertain the relative value of DCB compared with new-generation DES in different clinical and anatomic scenarios.
Authors’ perspective
Plain balloon angioplasty is used to predilate lesions and to optimise the results of stent implantation. Balloon angioplasty alone may be used in certain anatomic scenarios where stent implantation is not desirable. On the other hand, the value of DCB to safely and effectively deliver drug to the vessel wall has been demonstrated together with their clinical efficacy in both coronary and peripheral vessels. The value of DCB for patients presenting with ISR is very well established. Nevertheless, further studies are required to confirm their clinical value in selected “de novo” coronary lesions.
Conflict of interest statement
The authors have no conflicts of interest to declare. Bruno Scheller was named as co-inventor of two patent applications by Charite University Hospital, Berlin, Germany in 2001 and 2003.

