
Abstract
The first percutaneous coronary intervention (PCI) was performed in September 1977 by Andreas Grüntzig using a rudimentary balloon angioplasty catheter mounted on a fixed wire. PCI was immediately recognised as a potential breakthrough in cardiovascular medicine, but uptake in clinical practice was limited by unpredictable acute outcomes and a need for surgical standby. The introduction of bare metal stents (BMS) in the 1980s improved procedure reproducibility and clinical outcomes through a permanent scaffolding of the coronary vessel, preventing abrupt occlusion and acute recoil. It was the introduction of drug-eluting stents (DES) at the beginning of this century, however, that allowed PCI to become one of the most frequently performed therapeutic interventions in medicine, primarily by addressing the issue of in-stent restenosis. DES technology has improved considerably since, with iterative developments of the stent metallic backbone, the polymer coating, and the released antiproliferative agents impacting on the safety and efficacy profile of these devices in a meaningful way. Overall, the impressive technological advances in metallic coronary stents have revolutionised the treatment of ischaemic heart disease over the last 40 years. The aim of the present article is to provide an overview of past, present, and future aspects of coronary stent technologies.
The past
BALLOON ANGIOPLASTY
In 1977, Andreas Grüntzig pioneered the concept of percutaneous treatment of obstructive coronary lesions by means of balloon dilatation. This minimally invasive approach for the treatment of coronary artery disease was revolutionary, as it not only competed with coronary artery bypass surgery, avoiding open thoracotomy, but also emerged as the preferred treatment in the setting of acute myocardial infarction, replacing medical treatment with thrombolysis. However, the success of balloon angioplasty in clinical practice was limited by the risk of acute vessel recoil, negative vascular remodelling, restenosis and, in particular, the risk of abrupt vessel closure in the initial hours after the procedure.
BARE METAL STENTS
In 1986, Jacques Puel and Ulrich Sigwart introduced metallic coronary stents into clinical practice to overcome the key limitations of balloon angioplasty1. Bare metal stents (BMS) addressed one of the key limitations of balloon angioplasty by reducing the risk of abrupt vessel closure resulting from local dissections. In addition, the radial support of BMS eliminated the issue of vascular wall elastic recoil and constrictive remodelling observed after balloon angioplasty2. Indeed, two pivotal randomised trials – the Belgium Netherlands Stent Arterial Revascularization Therapies Study (BENESTENT) and the North American Stent Restenosis Study (STRESS) – showed a significant reduction in the incidence of angiographic restenosis and repeat revascularisation procedures with BMS as compared to balloon angioplasty in patients with stable CAD3,4. The optimisation of stent implantation techniques5 and the introduction of dual antiplatelet therapy – based on low-dose aspirin and a thienopyridine – as a substitute to oral anticoagulation after stenting6 favoured the diffusion of BMS use. In 1999, stent implantation was performed in more than 80% of percutaneous coronary interventions (PCI)7. Stent implantation, however, causes an arterial injury triggering vascular smooth muscle cell activation, migration, and proliferation, coupled with extracellular matrix formation, which ultimately results in the formation of neointimal tissue8. Excessive neointimal hyperplasia is the most common cause of in-stent restenosis and the need for repeat revascularisation. Indeed, this iatrogenic phenomenon occurred in up to 30% of patients treated with BMS and emerged as the key limitation of this technology. A number of alternative treatment strategies were proposed to address this issue, including systemic administration of antiproliferative agents, anti-inflammatory agents, alternative stent surface coatings, brachytherapy, intracoronary laser, and drug-eluting stents (DES)8.
DRUG-ELUTING STENTS
DES emerged in the early 2000s and were rapidly embraced due to the substantially improved antirestenotic efficacy of these devices as compared to BMS9-11. DES are based on three components, a metallic stent backbone, an antiproliferative agent, and a drug carrier (usually a polymer coating). These devices provide similar scaffolding properties to uncoated BMS, paralleled by site-specific release of antiproliferative agents that suppress neointima formation12. Indeed, in patients with straightforward lesions, CYPHER® sirolimus-eluting stents (Cordis [now Cardinal Health], Milpitas, CA, USA) were shown to virtually eliminate the risk of restenosis as compared to BMS in the RAVEL trial13. TAXUS™ paclitaxel-eluting stents (Boston Scientific, Marlborough, MA, USA) provided similar results in the TAXUS-IV trial14. A collaborative network meta-analysis including 38 randomised trials and over 18,000 patients indicated a pronounced benefit of DES as compared to BMS in terms of the risk of target lesion revascularisation, with a number needed to treat to prevent a revascularisation event of seven and eight patients treated with CYPHER and TAXUS stents, respectively15. Similar findings were observed in a comprehensive pairwise meta-analysis including 22 randomised trials and 34 observational studies with at least one year of follow-up, showing a consistent risk reduction for target vessel revascularisation in randomised trials (HR 0.45, 95% CI: 0.37-0.54) and observational studies (HR 0.54, 95% CI: 0.48-0.61) with DES as compared to BMS16.
In 2006, during presentations at the European Society of Cardiology annual meeting, concerns emerged regarding the long-term safety of DES, related in particular to an increased rate of (very) late stent thrombosis (ST) compared with BMS17-20. Although ST during the early post-implantation period was a well-recognised adverse event observed with BMS, this risk was mitigated by the introduction of dual antiplatelet therapy (DAPT) with aspirin and a thienopyridine in the initial weeks after stent implantation6. Systematic reviews and meta-analyses synthesising available evidence showed that the use of early-generation CYPHER sirolimus-eluting stents and TAXUS paclitaxel-eluting stents was associated with a small but steady risk of ST emerging beyond one year after stent implantation. This evidence led to the implementation of long-term (i.e., 12 months) DAPT after DES implantation21. The underlying pathophysiology was a delay in healing of the stented arterial segment with DES22. Of note, however, this hazard did not translate into a higher risk of death and myocardial infarction, which may be explained by the relatively low incidence of very late ST and the compensatory effects of DES antirestenotic properties19,23-26. New DES, developed since, addressed this issue in a meaningful way, further improving the safety and efficacy profile of DES therapy.
The present
CONTEMPORARY DRUG-ELUTING STENTS
New-generation DES are based on novel metallic alloys allowing thinner strut stent platforms and release drugs with improved antirestenotic effectiveness from new drug carriers. Figure 1 provides a schematic summary of the most diffuse DES technologies in Europe.
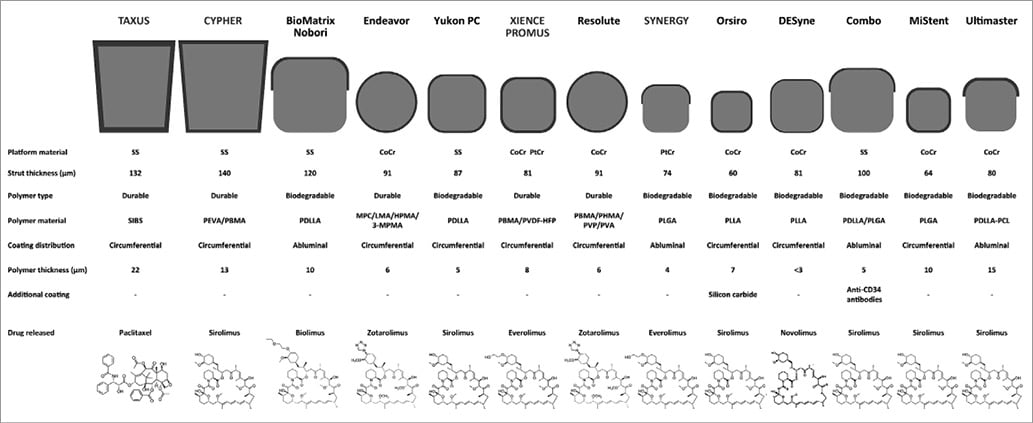
Figure 1. Progress of metallic drug-eluting stent technology. Metallic drug-eluting stents with durable or biodegradable polymer coatings. From top to bottom for each stent are reported: strut profiles, material features, and drug chemical formulas. CoCr: cobalt-chromium; HPMA: hydroxypropyl methacrylate; LMA: lauryl methacrylate; MPC: methacryloyloxyethyl phosphorylcholine; PEVA: poly-ethylene-co-vinyl acetate; PBMA: poly n-butyl methacrylate; PDLLA: poly-D, L-lactic acid; PHMA: polyhexyl methacrylate; PLGA: poly-lactic co-glycolic acid; PLLA: poly-L-lactic acid; PtCr: platinum-chromium; PVA: polyvinyl acetate; PVDF-HFP: co-polymer of vinylidene fluoride and hexafluoropropylene; PVP: polyvinyl pyrrolidinone; SIBS: poly(styrene-b-isobutylene-b-styrene); SS: stainless steel; 3-MPMA: trimethoxysilylpropyl methacrylate. Adapted from Mennuni M et al. Ann Biomed Eng. 201676 with permission.
As it relates to the stent platform, early-generation DES were based on a stainless steel platform with a strut thickness of 130-150 μm. Available evidence indicates that thinner stent struts (<100 μm) improve haemodynamic flow and drug distribution, favouring vessel healing at the stented site27,28. In addition, thinner stent struts elicit less vessel injury at implantation and have been shown to reduce restenosis in comparison with stents with thicker struts29. Moreover, stents with thinner struts appear to have a lower degree of thrombogenicity as compared to those with thicker struts. New-generation DES have backbones made of novel metallic alloys – such as cobalt-chromium and platinum-chromium – and have thinner stent struts (50-90 μm) while generally maintaining an adequate radial strength.
Drug carriers used for the release of antiproliferative agents in DES technologies are mostly comprised of polymeric materials adherent to the stent surface30. These polymer coatings allow an effective and controlled drug release at the stented site. However, once the antiproliferative drugs are completely released, the polymer coating is no longer required. Indeed, animal and pathology investigations – as well as intracoronary imaging-based clinical studies – have suggested that persistence of polymer coatings over time may trigger an inflammatory process within the arterial wall, resulting in an impaired healing of the stented artery31. With the aim of improving device biocompatibility, new-generation DES have been based on more biocompatible durable polymer coatings – such as the vinylidene-fluoride hexafluoropropylene copolymer – or biodegradable polymer coatings, composed of lactic or glycolic acids that fully resorb by hydrolysis after completion of drug release. Another approach to address the issue of biocompatibility is to eliminate the polymer coating completely32. Such technologies – known as polymer-free DES – release antiproliferative agents directly from the stent surface without the application of a polymer coating. A schematic summary of DES coating technologies is shown in Figure 2.
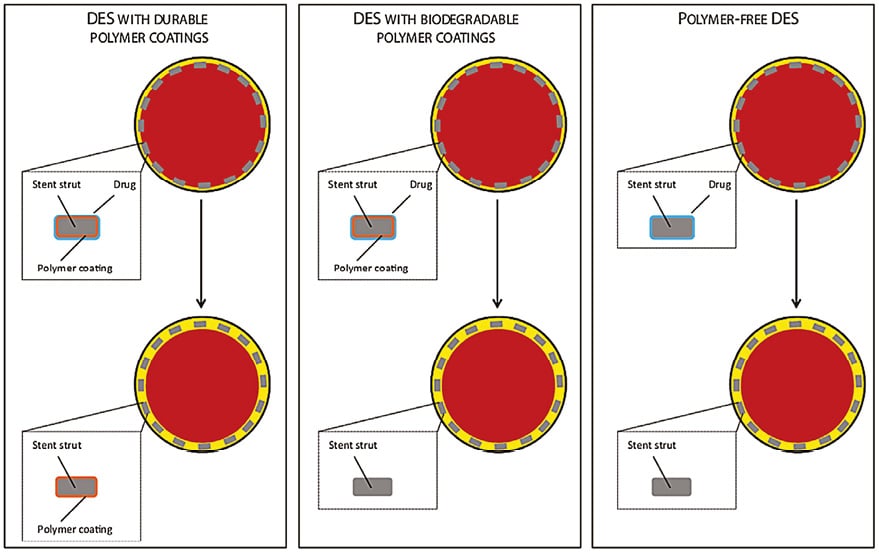
Figure 2. Drug-eluting stent technologies. The following DES technologies are summarised: DES with durable polymer coatings, DES with biodegradable polymer coatings, and polymer-free DES. The top panels depict coronary cross-sections and stent cross-sections at the time of implantation. The bottom panels depict the same features after complete drug release. The vessel lumen is displayed in red, the intima in yellow, and the stent struts in grey. Adapted from Stefanini GG et al. Heart. 201430 with permission.
Regarding antiproliferative drugs, paclitaxel and sirolimus were those used on early-generation DES platforms. Paclitaxel interferes with microtubule dynamics during mitosis by binding to the β-tubulin subunit of microtubules33. Sirolimus blocks protein synthesis, cell cycle progression, and cell migration by inhibiting the mammalian target of rapamycin34. The antirestenotic efficacy of sirolimus-eluting DES has been consistently shown to be higher than that of paclitaxel-eluting DES35. This may be due to different tissue kinetics between the drugs and a wider therapeutic index with sirolimus. Because of this, new-generation DES use sirolimus (or its analogues such as everolimus, zotarolimus, biolimus, and novolimus, which differ from each other in terms of structure, molecular weight, potency and lipophilicity).
CLINICAL EFFICACY
The clinical efficacy of coronary stents is typically measured by freedom from in-stent restenosis and the subsequent need for repeat revascularisation procedures over time36. Contemporary new-generation DES have been shown to improve upon the efficacy profile of early-generation devices.
The XIENCE/Promus durable polymer-based everolimus-eluting stent (DP-EES) has perhaps the broadest body of evidence from trials. Several other new-generation DES have been approved for use in Europe and investigated in large-scale randomised clinical trials. These include the Resolute™ durable polymer-based zotarolimus-eluting stent (Medtronic, Dublin, Ireland), the BioMatrix™ (Biosensors, Singapore) and Nobori® (Terumo Corp., Tokyo, Japan) biodegradable polymer-based biolimus-eluting stents, the Orsiro (Biotronik, Bülach, Switzerland) biodegradable polymer-based sirolimus-eluting stent, the SYNERGY™ (Boston Scientific) biodegradable polymer-based everolimus-eluting stent, the Ultimaster® (Terumo Corp.) biodegradable polymer-based sirolimus-eluting stent, and two polymer-free DES – the Coroflex® ISAR (B. Braun, Melsungen, Germany) sirolimus-eluting stent and the BioFreedom™ (Biosensors) biolimus-eluting stent. A number of observational studies37, randomised trials38-40, meta-analyses41,42, pooled analyses of individual patient data43, and network meta-analyses44,45 have confirmed the significantly superior efficacy of new-generation DES as compared to BMS and early-generation DES. A comprehensive synthesis of available randomised evidence was provided in a systematic review by the Task Force on coronary stent evaluation of the European Society of Cardiology (ESC) and European Association for Percutaneous Cardiovascular Interventions (EAPCI)11. The analysis included a total of 158 randomised trials and showed a progressive improvement in efficacy associated with device iteration. As summarised in Figure 3, early-generation DES were associated with a lower risk of target lesion revascularisation as compared with BMS, and new-generation DES provided a further risk reduction (median rates per 100 person-years at 12 months: BMS 12.3%, early-generation DES 4.3%, new-generation DES 2.9%)11.
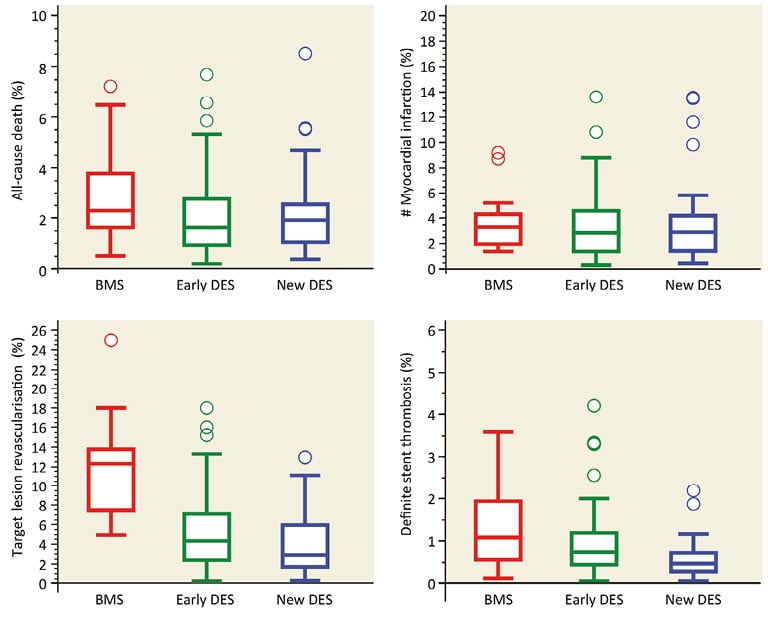
Figure 3. Progress in clinical efficacy and safety associated with device iteration. Results of a systematic review including 158 randomised trials. The Figure shows median rates and interquartile ranges per 100 person-years at 9 to 12 months with BMS (red), early-generation DES (green), and new-generation DES (blue) for the clinical endpoints all-cause death, myocardial infarction, target lesion revascularisation, and definite stent thrombosis. Adapted from Byrne RA et al. Eur Heart J. 201511 with permission.
CLINICAL SAFETY
The clinical safety of coronary stents is measured by freedom from coronary ischaemic events over time36, including patient-specific outcomes such as death and myocardial infarction as well as device-specific outcomes such as ST. Technological improvements in new-generation DES have translated into an improved safety profile of these devices as compared to early-generation DES. In particular, the issue of (very late) ST has been resolved. Indeed, several large-scale observational studies37,46,47, randomised trials38-40,48, meta-analyses41,42, pooled analyses of individual patient data43, and network meta-analyses44,45 have consistently shown the superior safety profile of contemporary new-generation DES as compared with early-generation DES. A network meta-analysis of 49 randomised trials provided some evidence that new-generation DES – specifically DP-EES – might be associated with a lower risk of ST as compared with BMS (OR 0.24, 95% CI: 0.14-0.43)44. This finding was confirmed in a patient-level meta-analysis of 4,896 patients from five randomised trials directly comparing DP-EES with BMS. At two-year follow-up, patients treated with DP-EES had a lower risk of definite ST (HR 0.41, 95% CI: 0.22-0.76; p=0.005) and definite or probable ST (HR 0.48, 95% CI: 0.31-0.73; p<0.001), paralleled by a lower risk of cardiac mortality (HR 0.67, 95% CI: 0.49-0.91; p=0.01) and myocardial infarction (HR 0.71, 95% CI: 0.55-0.92; p=0.01). Moreover, in the large-scale NORSTENT trial – which directly compared DES with BMS in 9,013 patients40 – DES were associated with a lower risk of ST as compared to BMS (0.8% vs. 1.2%; HR 0.64, 95% CI: 0.41-1.00, p=0.0498) at six-year follow-up. The improved safety performance of new-generation DES as compared to early DES and BMS was also observed in the systematic review on coronary stents of the ESC-EAPCI Task Force (Figure 3)11. Of note, this resulted in an attenuation of the benefit of prolonged DAPT for ST prevention after new-generation DES implantation49. Whether the improved device safety profile translates into a reduced risk of hard ischaemic events – such as death and myocardial infarction – remains a matter of debate. The NORSTENT trial did not show any significant differences between DES (mostly new-generation) and BMS for the composite primary endpoint of death and myocardial infarction up to six-year follow-up (16.6% vs. 17.1%; HR 0.98, 95% CI: 0.88-1.09, p=0.66)40. Conversely, a network meta-analysis evaluating myocardial revascularisation strategies as compared with medical therapy alone in patients with stable CAD showed that new-generation DES might be associated with a significant reduction in the risk of mortality that was not observed with earlier devices50. Finally, new-generation DES have significantly narrowed the gap between PCI and coronary artery bypass grafting (CABG) for the treatment of multivessel CAD and left main disease. As it relates to the latter, the recently published EXCEL trial demonstrated non-inferiority of PCI with new-generation DP-EES as compared to CABG in patients with low-to-moderate anatomical complexity of CAD (i.e., SYNTAX score <33) with respect to the composite of death, stroke, and MI at three-year follow-up51. This was partly in contrast to the findings of the NOBLE trial, which failed to show non-inferiority between PCI with biodegradable polymer-based biolimus-eluting stents and CABG in patients with left main disease – irrespective of anatomical complexity of CAD – for the composite of death, MI, stroke, and any repeat coronary revascularisation, while suggesting improved outcomes with CABG at five years of follow-up52. Both EXCEL and NOBLE were included in a meta-analysis of five trials including 4,595 patients with left main disease randomly allocated to PCI with DES or CABG. While suffering from the limitation of pooling average aggregate treatment effects on the study level without the possibility to stratify the population by CAD anatomical complexity and other relevant comorbidities, the findings of this meta-analysis showed similar risks with PCI and CABG in terms of all-cause mortality (OR 1.01, 95% CI: 0.76-1.34), cardiovascular mortality (OR 1.02, 95% CI: 0.73-1.42), MI (OR 1.45, 95% CI: 0.87-2.40) and stroke (OR 0.87, 95% CI: 0.38-1.98) at a median follow-up of 60 months53.
The future
Based on the available evidence summarised in this article, contemporary metallic DES are characterised by an excellent safety and efficacy profile and represent the standard of care for patients undergoing PCI at this point in time. Notwithstanding this, an unmet need can be said to exist, with some subgroups of clinically or anatomically complex patients still showing relatively high adverse event rates after stenting. Moreover, despite the excellent performance of contemporary DES, the persistent but superfluous metallic components represent a potential nidus for adverse events in the years after implantation, an issue of particular relevance in otherwise healthy young or middle-aged patients with long life expectancy.
HIGH BLEEDING RISK
Dual antiplatelet therapy is recommended for patients with CAD undergoing PCI with DES implantation54. The optimal duration of DAPT, however, still represents a matter of debate. Several recent lines of evidence indicate that DAPT duration should be individualised based on a risk-benefit evaluation taking into account patients’ risk profiles and comorbidities55,56. Of note, the ageing of the population observed over the last decade means that more and more elderly patients are presenting with symptomatic obstructive coronary disease. Such patients often have multiple comorbidities and an increased risk of bleeding. Therefore, reducing the required duration of DAPT is a pivotal goal of future DES technologies. The LEADERS FREE trial has shown polymer-free biolimus-eluting stents to be safe and effective in patients at high bleeding risk treated with one-month DAPT. However, the comparator device in that trial was a BMS, rather than a new-generation DES, which is now regarded as the standard of care. Indeed, several studies are currently evaluating a one-month or shorter DAPT duration after the implantation of other new-generation DES (clinicaltrials.gov: NCT03023020, NCT03112707, NCT02594501). In the future, high-performance new-generation DES implanted with optimal technique and effective periprocedural antithrombotic therapy may not require DAPT for the prevention of ST. The choice of antiplatelet therapy may be guided more by the general patient risk profile – with the aim of reducing the global atherothrombosis risk – rather than by the need to prevent thrombotic complications within the implanted device.
ACUTE MYOCARDIAL INFARCTION
One important advance in cardiovascular medicine is the widespread implantation of primary PCI over thrombolysis, resulting in faster, more effective and durable reperfusion, reduced infarct size, lower rates of intracranial haemorrhage and improved survival54. The ESC guidelines on myocardial revascularisation recommend mechanical reperfusion with primary PCI and DES implantation in patients with acute myocardial infarction (AMI)54, based on the improved efficacy of new-generation DES as compared with early-generation DES and BMS without any safety concerns57-59. Notwithstanding this, patients with AMI are characterised by higher rates of stent-specific adverse events as compared to patients with stable CAD60. This may be partly explained by plaque instability of AMI culprit lesions. Moreover, suboptimal stent sizing and subsequent malapposition represent another important determinant of increased stent-related events in AMI patients. This occurs as a consequence of coronary vasoconstriction as well as thrombus presence at the culprit lesion site. Self-expanding coronary stents have been proposed to address this problem, although large outcome trials are missing at this point in time61. In order to reduce the risk of distal embolisation in the acute setting, mesh-covered stents have been developed with the aim of immobilising atherothrombotic material at the site of stent implantation (i.e., between the stent and the vessel wall)62.
DIABETES MELLITUS
Patients with diabetes mellitus are characterised by more advanced and complex CAD, with long lesions involving multiple vessels, bifurcations, and small coronary arteries63. Unsurprisingly, diabetes status remains an independent predictor of impaired clinical outcomes in patients undergoing PCI despite the use of new-generation DES64. New-generation DES have been shown to improve safety and efficacy outcomes as compared to early-generation DES65,66. However, the favourable treatment effects of new-generation DES compared with early-generation DES appear partly attenuated in patients with diabetes mellitus as compared to those without66-68. Clinical outcomes of diabetic patients might, therefore, be improved by further iterations in DES technologies aiming to increase antirestenotic potency while maintaining a favourable biocompatibility profile. A dedicated DES eluting sirolimus from a matrix coating combining an active drug with fatty acids has shown some promise in patients with diabetes69 but requires further investigation in large-scale randomised trials.
BIFURCATION LESIONS
The optimal treatment strategy for bifurcation lesions remains a matter of some debate70,71. Although provisional stenting of the main branch followed by stenting of the side branch only if required is generally the preferred approach, results may be suboptimal in a significant proportion of cases72. Against this, systematic stenting of both the main vessel and the side branch – a two-stent strategy – may lead to higher risk of restenosis and thrombotic events triggered by the overlapping stent layers72. In order to optimise treatment of bifurcation lesions, a number of dedicated stents have been proposed72. Among several technologies developed for the treatment of bifurcations, the Tryton stent (Tryton Medical, Inc., Durham, NC, USA) is the only device that has been directly compared with standard stenting in the setting of a randomised clinical trial. This device, however, is limited by lack of drug elution and it failed to show benefit over conventional bifurcation stenting in a recent randomised trial, with higher rates of myocardial infarction as compared to standard stenting73. It must be acknowledged that the variability across possible bifurcation anatomies poses a significant challenge to the development of dedicated bifurcation stent technologies and further iterative development is needed.
RESTORATION OF VASCULAR PHYSIOLOGY
An intrinsic limitation of metallic DES is represented by leaving a permanent implant caging the stented segment. The persistence of a metallic prosthesis within the treated vessel may prevent the restoration of full vascular physiological functions which may be important in case of very long lesions and the ability to bypass diseased segments in the future74. Fully bioresorbable coronary scaffolds have been developed to address these limitations. These devices provide temporary scaffolding properties through a polymeric or metallic bioresorbable backbone that completely resorbs after completion of drug release. Evaluations of this technology so far have provided evidence of complete stent resorption, restoration of vasomotion and cyclic strain within the treated coronary segment, as well as positive vessel remodelling75. However, there have also been reports raising concerns regarding the long-term resorption process including scaffold discontinuities. Clinical trials on currently available bioresorbable scaffolds indicate that additional iterations will be needed for the technology to provide a safety and efficacy profile comparable to contemporary metallic DES77. Whether the potential advantages of vascular physiology restoration may translate into clinically meaningful benefits will need to be evaluated in appropriately designed studies.
Conclusions
The field of PCI has been subject to impressive technological advances since the first balloon angioplasty of Andreas Grüntzig in 1977. The development and optimisation of coronary artery stent technologies has played a key role. Balloon angioplasty was superseded by BMS that addressed the issues of acute recoil and abrupt vessel occlusion by permanently scaffolding the treated vessel. Restenosis – the principal limitation of BMS – was, in turn, addressed by DES with a subsequent substantial improvement in clinical outcomes. This permitted a broader adoption of PCI to higher-risk patient and lesion subsets, which had previously been associated with a prohibitive risk of restenosis after stenting. Iterative development of DES technologies led to devices made of new metallic alloys allowing thinner stent struts and releasing limus antiproliferative agents from more biocompatible durable or biodegradable polymer coatings. These new-generation DES have provided improvement to the safety profile of earlier-generation DES combined with an improved overall antirestenotic efficacy. Contemporary DES provide excellent safety and efficacy outcomes, are the standard of care for patients undergoing PCI, and should represent the benchmark to evaluate any novel coronary device.
Authors’ perspective
While contemporary metallic DES are characterised by an excellent safety and efficacy profile, specific patient populations remain at higher risk of adverse events due to their clinical and anatomical complexity. Moreover, the need for DAPT to prevent ST is still perceived as a potential limitation of metallic stents. Future metallic DES technologies should address the need to improve outcomes among high-risk subgroups such as patients with AMI, diabetes mellitus, diffuse coronary artery disease and bifurcation lesions. In addition, future DES should be characterised by minimal device thrombogenicity, with the ultimate goal of basing the selection of antithrombotic regimens on the individual patient risk profile rather than on the prevention of ST.
Conflict of interest statement
G. Stefanini has received a research grant (to the institution) from Boston Scientific, and speaker/consultant fees from Boston Scientific, B. Braun, Biosensors, and Edwards Lifesciences. R. Byrne reports personal fees from B. Braun Melsungen AG, Biotronik, and Boston Scientific, and research grants from Boston Scientific and HeartFlow. S. Windecker has received grants to the institution from Biotronik, Boston Scientific, Bracco Pharmaceuticals, Edwards Lifesciences, Medtronic, St. Jude Medical and Abbott Vascular, outside the submitted work. A. Kastrati has a patent EP1402849 stent with rough surface licensed to Translumina Therapeutics LLP, India, a patent EP2073856 coated implant licensed to Translumina Therapeutics LLP, India, and a patent EP1933893 implant with multiple coating licensed to B. Braun, Germany.

