Background
The introduction of stents in the early 1990s was a major advancement in combating restenosis post coronary intervention. The implantation of a stent reduced the incidence of restenosis to roughly 20% to 30% as compared to balloon angioplasty in which restenosis of the treated segment typically occurred within six months in about 40% of cases1,2. The use of stents eliminated the loss of vessel lumen which occurred secondary to acute vessel recoil and late negative or pathological remodelling previously seen in balloon angioplasty. However, in doing so, stents also eliminated the process of late adaptive or expansive remodelling which over time, advantageously contributed to luminal gain in patients3. Currently stents are used in over 75% of all interventional procedures worldwide4.
Subsequent to the development of the initial stent designs, researchers continued to look for ways to further reduce restenosis rates. While stents prevented lumen loss secondary to recoil and negative remodelling, they did not prevent restenosis due to neointimal hyperplasia. The next major advancement came in the form of a combination device, the drug eluting stent (DES). These metal stents commonly combine a thin polymer coating and therapeutic drug that significantly inhibits neointimal hyperplasia after stent implantation; thus further reducing the incidence of restenosis5,6.
Delivery of drugs locally to the artery wall, using significantly lower dosages than systemic applications, has been shown to inhibit smooth muscle cell migration and proliferation, which is considered a major component in the cascade of events leading to neointimal hyperplasia and restenosis. Drug eluting stents currently remain the most utilised stents today due to their ability to significantly reduce restenosis as well as in-stent restenosis7,8. The most recent DES clinical trials demonstrate a restenosis rate of 5-10%.
In spite of these significant advancements, the use of metal-based drug eluting stents that remain permanently embedded within the arterial wall still represents a number of potential drawbacks.
These drawbacks include9,10:
– predisposition to late stent thrombosis,
– prevention of late vessel adaptive or expansive remodelling,
– hindrance of surgical revascularisation,
– impairment of imaging with multislice CT.
In an effort to continually improve outcomes in interventional cardiology patient care, researchers are pursuing novel approaches to solve the drawbacks described above while preserving the advances offered by current metallic stents and DES. One such effort has come in the form of fully bioresorbable stents that act as “temporary” scaffolds to support the vessel during the initial critical months of healing and remodelling after dilation. Such an approach has the potential to provide the benefits of metal stents – prevention of acute vessel recoil and late negative or pathological remodelling – without the permanency. In addition, if the bioresorbable stent was drug-eluting, it could offer the additional benefit of a traditional metallic DES by inhibiting neointimal hyperplasia.
Previous studies in both animal models and clinical cases have documented that the most critical period of vessel remodelling post intervention is largely complete by approximately three months11,12. Thus, the goal of a bioresorbable or “temporary” scaffold should be to fully support the vessel during this critical period while potentially allowing for late adaptive or expansive remodelling and subsequent luminal gain.
Initial efforts focused on developing a fully bioresorbable stent technology, have proven to be difficult at best. There is a fundamental dilemma in attempting to adapt current metal stent designs to a platform consisting of a bioresorbable material. Conventional stent designs are material-specific in that they rely on materials that can undergo significant shape alterations (metal deformation) from balloon pressure and expansion in order to enlarge and support a vessel. Due to the rigidity and non-elastic nature of bioresorbable materials new innovative solutions are required to overcome this dilemma.
The REVA approach
Slide & Lock design
From the beginning, REVA took a unique approach to fully integrate both a novel design and material, in developing a stent that provides the benefits of metal without the permanency. The desired performance benefits include adequate strength both at implant and over time, traditional (and expected) expansion range as well as device visibility.
Unlike traditional deformable metal stent designs, the REVA Slide & Lock design (Figure 1) deploys by sliding open and locking into place13,14. This novel stent design completely eliminates the need to deform the device, making it ideally suited to use with polymers, which are inherently not as amenable to deformation as metals.

Figure 1. REVA Side and Lock design elements.
The Slide & Lock design provides significant advantages when developing a bioresorbable stent. It prevents the deformation that typically weakens a polymer device as it expands. By “locking in” the diameter the stent maintains the acute lumen gain (i.e., no traditional acute recoil), maintains support through the healing process and allows for a range of diameters with a single stent size. For example, the 3.0 diameter stent can treat vessels over a 2.9 to 3.4 mm range.
This unique Slide & Lock design allows both the strength and the flexibility of the stent to be tuned and optimised independently. Conventional, deformable stent designs typically require designers to compromise on one, or both of these features. In a conventional stent design, as the thickness is increased to increase strength, the flexibility of the design simultaneously decreases and it becomes harder to bend the thicker material. The REVA stent design leverages design features that allows flexibility to be maintained even when thicker, stronger materials are employed.
Inherently radiopaque tyrosine-derived polycarbonate material
In cooperation with Professor Joachim Kohn, at Rutgers University in New Jersey, USA, REVA has developed a unique and proprietary iodinated poly(DTE carbonate) material to serve as the base polymer for the device. Desaminotyrosyl-tyrosine ethyl ester (DTE) is a member of the family of desaminotyrosyl-tyrosine alkyl esters. The formulation of this material provides REVA with the ability to adjust the degradation profile to fit the desired application. REVA’s polymer has been engineered to have a degradation profile that would support the vessel during the initial healing and remodelling process prior to significant degradation of the stent.
A major drawback of contemporary polymers is that they lack intrinsic radiopacity. An important advancement in the development of this new material has been the enhancement of x-ray visibility. Iodine atoms are covalently bound directly to the backbone poly(DTE carbonate) matrix. Iodine atoms, due to their greater mass, scatter x-rays and impart radiopaqueness15-17. The incorporation of this material into the polymer backbone allows the REVA Bioresorbable Coronary Stent to be visualised using the exact angiographic techniques that are currently used by physicians today (Figure 2).
Figure 2. Radiopacity of the REVA Bioresorbable Coronary Stent shortly after implant in a porcine animal model.
Stent performance: acute radial strength
Acute radial strength of the stent is important to maintain initial luminal gain and prevent acute vessel recoil. As seen in Figure 3, geometric design and polymer selection of the REVA stent provides acute radial strength comparable to contemporary metal stents.
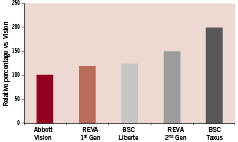
Figure 3. Relative percentage of acute radial strength of metal and polymer stents versus the Vision stent. Test adapted from Kalmar G, et al 200219.
Stent performance: balancing strength and bioresorption
The balance between adequate scaffold support and bioresorption is unique to polymer-based stents. The process of resorption begins with a loss in molecular weight as the material takes in water and begins to break down via hydrolysis. The loss of molecular weight corresponds to a loss of mechanical strength of the device. As seen in Figure 4, the REVA stent is specifically engineered to maintain at least 75% of its initial mechanical strength to support the vessel through the most critical 3-month healing and remodelling phase, but to no longer constrain the vessel and allow for the potential of expansive remodelling after 6-12 months.
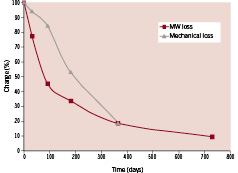
Figure 4. Graph showing the changes in stent materials after in vitro degradation. Testing included a hydrolytic radial crush test (pressure to crush) and gel permeation chromatography to measure the weight average molecular weight in kilodaltons. All data are shown as a percentage relative to their time zero control.
Stent performance: bioresorption to allow expansive remodelling
As stated, the goal of the REVA program is to develop a stent that provides the performance benefits of metal without the permanency. While preserving metal’s benefits of strength and visibility, perhaps one of the most promising aspects of bioresorbability is the opportunity to allow the vessel, and not the stent, to define and restore the optimal lumen. In the case of a metal stent, the lumen is defined and fixed by the stent itself, thus preventing the process of late adaptive or expansive remodelling. In contrast, with a bioresorbable device, as the stent resorbs, the lumen size can be optimally restored by the vessel and expanded to more closely match non-diseased/non-stented vessel segments. As seen in Figure 5, the REVA stent allows for this late adaptive or expansive remodelling with subsequent luminal gains at 6 and 12 months in animal models.
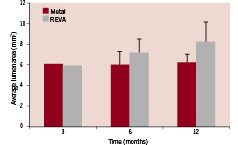
Figure 5. Average luminal area versus time for metal control and the REVA bioresorbable stent as measured by intravascular ultrasound (IVUS). IVUS measurements were performed by the IVUS Core Lab at the Jack H. Skirball Center for Cardiovascular Research in Orangeburg, NY, USA under the direction of Dr. Greg Kaluza, MD.
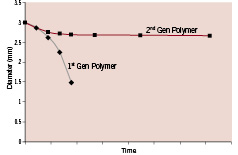
Figure 6. Performance comparison of the original and modified polymers under load. Deployed stents were evaluated under cyclic loading under accelerated time-to-failure conditions in phosphate buffered saline (PBS) at 37°C.
Initial clinical experience
The first-generation REVA Bioresorbable Coronary Stent was evaluated clinically in the REVA Endovascular Study Of a BioResorBable Coronary Stent (RESORB) Study18.
The primary objective of the study was to demonstrate the 30-day safety of native coronary artery stenting using the REVA Bioresorbable Coronary Stent by assessing the 30-day event free survival rate (MACE). The secondary objectives were to assess the performance (procedural and technical success) of the device, the safety outcomes (MACE) through 60 months and the 6-month Quantitative Coronary Angiographic (QCA) & IVUS derived parameters.
This was a prospective, multi-centre, safety study with a study population consisting of a maximum of 30 patients with clinical evidence of myocardial ischemia or a positive functional study. Patients with single de novo lesions in native coronary vessels ranging in diameter from 3.0 to 3.4 mm and with a lesion length up to 12 mm were to be included.
REVA implanted the first generation bioresorbable stent in 25 patients in the RESORB trial.
The average diameter stenosis (DS) treated was 70%, and the device was able to successfully hold good acute gain (MLD (mm) was 0.88±0.39; 70% DS pre-implant and 2.76±0.36; 5.9% DS post-implant). Moreover, the device successfully prevented pathological or negative remodelling, as defined by the maintenance of the external elastic lamina (EEL) during the initial healing and remodelling phase. The EEL at implant compared to follow-up (4–6 months) was nearly identical (15.5±4.0 mm2 vs. 15.3±3.1 mm2).
The device was also able to treat a variety of lesion types (16% Type A, 48% Type B1, 28% B2, 8% Type C) and sizes (2.7 mm to 3.3 mm) with a 3.0 mm diameter stent.
Although acute performance of the stent was promising, a higher than anticipated target lesion revascularisation rate occurred between four and six months post implant.
The future
The results of the first clinical experience demonstrated that further polymer and design optimisation was required. The stent needed to perform as intended, not only at the bench and in contemporary industry-standard pre-clinical models, but under dynamic, “real life” clinical loads. Moreover, bench testing and preclinical models that are more predictive of the clinical situation are required when developing a bioresorbable stent.
To achieve these goals, a minor modification was made to the first-generation polymer to improve its performance under load. Figure 6 denotes a comparison of the performance of the original and modified polymers under load (as measured by diameter change over time). The results demonstrated that the modified polymer exhibited enhanced dimensional stability and maintained mechanical integrity under severe loading for an extended period of time.
Confirming bench results in vivo
To further assess the “real life” fatigue performance of the optimised polymer, an in vivo partial stent overlap test was utilised. Polymeric stents were deployed into porcine coronary arteries followed by the partially overlapped deployment of a metal stent. The overlap-stented segments were examined one month post-implant. The radiographs shown in Figure 7 demonstrate the ability of the optimised 2nd generation polymer to maintain structural integrity under harsh in vivo conditions.
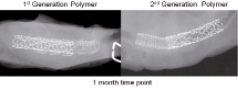
Figure 7. Faxitron x-ray images of the excised partially overlapped stents. Pathology by Dr. Fred J. Clubb, Jr., Cardiovascular Pathology Research, Texas Heart Institute, Houston, TX.
The optimised polymer has been fully integrated with the next-generation Slide & Lock design (Figure 8) and combined with delivery of a ‘-limus drug. The Company anticipates re-entry into the clinic with the ReZolve™ drug-eluting bioresorbable coronary stent in early 2010.
Figure 8. REVA’s ReZolve™ bioresorbable drug-eluting coronary stent.

