Coronary angioplasty (originally abbreviated PTCA, later PCI) was officially launched in September 1977. It is often forgotten that this revolutionary revascularisation technique has a number of forefathers who laid the grounds for all percutaneous endeavours.
Among the pioneers that made angioplasty and other interventional techniques possible, Konrad Röntgen, whose wife lent her hand to be photographed with X-rays 120 years ago, played a particular role. In 1929, Werner Forssmann pushed a catheter down his own left arm into the right atrium. His wish at that time was to eject drugs directly into the heart. While he was not thinking of mechanical probing, pushing or drilling, he launched the idea of using catheters for other therapeutic tasks. The Nobel Prize Committee rewarded him in 1956 with it’s prize for his work.
Next in line, and probably the most important pioneer of angioplasty, is Charles Dotter. Charles Dotter theorised that he could pass a type of rod through any occlusion or any very tight lesion...which, as we know, is exactly what he did for the first time in January 1964 when a surgeon sent him a patient with occlusive disease of the superficial femoral artery, which he considered not treatable by surgery.
Andreas Grüntzig modified this technique by making the rod variable in dimension by using sausage like balloons, which could be inflated when in place. Retrospectively, he was overly optimistic regarding the composition of the plaque, which he used to compare to “wet snow“.
In 1978, I started adopting the method shortly after Andreas Grüntzig did his first case, performing my own first cases in Bad Oeynhausen, Germany. In 1979, I moved to Lausanne, Switzerland, continuing my work which revealed two fundamental problems:
1) Only proximal lesions could be attained.
2) The outcome after the use of force to widen the lesion was mostly unpredictable.
Figures 1 and 2 say more than a thousand words. A proximal LAD stenosis is seen before –and immediately after– balloon dilatation. Careful inspection shows an obvious dissection line inside the lesion.
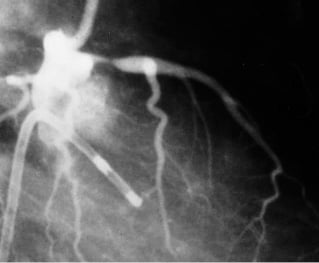
Figure 1.A proximal LAD stenosis.
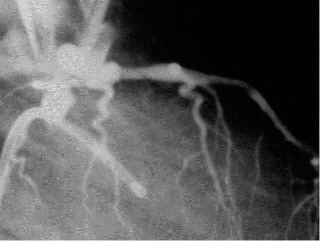
Figure 2.A proximal LAD stenosis after balloon dilatation. Careful inspection shows an obvious dissection line inside the lesion.
It was this unsatisfactory outcome which called for intravascular scaffolding.
In 1977 the “positive” outcome of balloon angioplasty for single lesions was in the order of 80%; with 4.5% ending up with myocardial infarction, 2.5 % with emergency surgery. There was a 30-40% clinical recurrence rate.
In 2010 things have changed. The single lesion success has increased to over 98%, the AMI rate has decreased to less than 1%, emergency surgery has decreased to less than 1% and the angiographic recurrence rate as well has decreased to less than 5%.
What are the factors that made this evolution possible?
In the mid-eighties the idea of using some structural support came to my mind. After long hours of brainstorming, we settled for the self-expanding mesh stent which we developed together with asmall company in Lausanne called Medinvent.
In 1986, the indications for stenting as proposed to the ethics committee were as follows:
– Abrupt closure after angioplasty
– Restenosis after angioplasty
– Lesions in saphenous vein graft
The first proof of concept, and a historical breakthrough, occurred in 1986, where we dilated a right coronary artery subtotal stenosis during a live course in Lausanne, followed by a left coronary artery (LAD) tandem lesion. After the intervention we found the patient was in pain and discovered that the LAD was totally closed. We discussed this with our surgeons and placed a stent across the occlusion. The patient became immediately pain free, and has had a good quality if life up until today.
The four main problems after placing of stents are the following:
– thrombus,
– inflammation
– hyperplasia
– remodelling
Thrombosis continued to be a severe problem, causing the device industry to back away from concentrating on stents for a certain period of time. The answer to this problem was the inhibition of platelets by the combination of aspirin and thienopyridines.
Hyperplasia can to some degree be controlled by reducing strut thickness. A comparative series showed that stents with 50 µm strut thickness produced 17.9 % restenosis, whereas in those with 140µm strut thickness, the amount of hyperplasia is much more important (31.4%)1.
The most effective way to suppress hyperplasia is using certain coatings which release antimitotic drugs. These coatings are not always biocompatible, and in most instances invariably create inflammatory reactions, no matter which of the coatings are used. It has become clear that the problem of thrombosis persists for years after implantation, and the very late stent thrombosis rate remains, today, at about 0.5% per year2.
Despite all these side-effects, the use of second generation stents has brought us rather satisfactory outcomes after coronary angioplasty.
Meanwhile, a third generation of stents (bioabsorbable coronary stents) has been developed, which again have their own specific problems:
1. The inflammatory reaction is pronounced
2. They are too bulky
3. Stent fractures at minor overstretch can be seen
4. There is insufficient radial force with these plastic stents
With bioabsorbable metal stents, target vessel revascularisation is still at 45%3. The latest generation of bioabsorbable plastic stents come with higher radial strength, and less inflammatory reactions. Recent publications claim a binary restenosis rate of only 2.4%, and a late loss that is perfectly acceptable4. In one recent series with asmall number of patients (45/45), there was literally no thrombosis at all5.
At this point in time, it seems as if the everolimus-eluting bioabsorbable plastic stents have a number of advantages:
– less inflammation
– easy placement
– adequate radial strength
– minimal recoil
– no stent thrombosis (until now)
– preservation of vasomotion and endothelial function
– probability of being fully resorbed in two years6
Vanishing stents may become a practical solution to keeping arteries open, without the long-term problems which persist due to the placement of hardware in coronary arteries.
In summary (Figure 3), we can see that over the last 30 years, while failures in angioplasty have not completely disappeared, the technique itself has improved significantly and continues to do so. Emergency coronary artery grafting is a dying art. Restenosis has come down to single digits, and, with the use of drug-eluting stents, the rate of acute stent thrombosis has become acceptable. It is the admittedly low, very late stent thrombosis which remains worrisome, and vanishing stents may, in the future, overcome this problem.
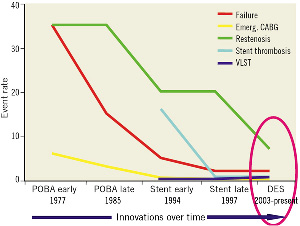
Figure 3. Thirty years in the evolution of PCI (adapted from Donald Baim).
Conflict of interest statement
The author has no conflict of interest to declare.
References

