
Performing coronary angioplasty
Andreas Roland Grüntzig (1939-1985) was an accomplished clinician and an astute scientist. He was also a practical man endowed with dexterity, smartness, and common sense. For him, performing a catheter intervention in contrast to surgery was like playing the clarinet in contrast to playing the piano. It was easier but it still required talent and proper training to become a professional. Playing the piano means using all ten fingers at the same time and having 88 keys to choose from for every single finger, not to mention the simultaneous work on the foot pedals. A clarinet also requires the use of all ten fingers but each finger has just a single (exceptionally up to five) allotted function. Clarinet players may forgive my not mentioning the importance of the mouthpiece. The analogy is just to make a point. The cardiac surgeon works three-dimensionally with every stitch, every cut, and every suture having to meet quality requirements and representing his or her level of art. Performing a catheter intervention, on the other hand, only permits one to advance, retract, turn right, or turn left, one, two, or at the most three different instruments at a time. Yet, while the surgeon has a true three-dimensional field of vision, a catheter operator has to imagine the third dimension looking at a two-dimensional black and white picture. The surgeon approaches things directly and one millimetre (mm) of motion equals 1 mm of effect. The catheter operator has to account for a time delay of his motion and a five centimetre movement at his end of the catheter may well translate into no or just a very small movement at the other end of the catheter inside the patient. Video gaming may come close to what an operator experiences during catheter interventions. Grüntzig, living before the video game era, trained with catheters on the kitchen table and started performing his intervention in the leg where inaccuracy and imperfection were more forgivable and less dangerous.
While working in the leg, it was convenient to use a torquable guidewire with a J-tip to steer away from obstacles and to target the right direction in bifurcations. Grüntzig had to give that up when he turned to coronary angioplasty1. In the small catheter shaft required for coronary work, technology at the time did not allow a through lumen for a steerable guidewire and at the same time two additional lumina, one for balloon filling and one for distal pressure measurements. Fluoroscopy visibility at the time was so poor (Figure 1) that it was mandatory to guide the procedure by distal pressure measurements (Figure 2). Instead of a steerable guidewire, a short wire stub attached at the tip of the catheter had to be accepted as a compromise (Figure 3). The original balloon was quite compliant and ruptured at about 6 bar. Other than that, and in spite of the lack of steerability, this balloon could still be used nowadays for simple proximal lesions.
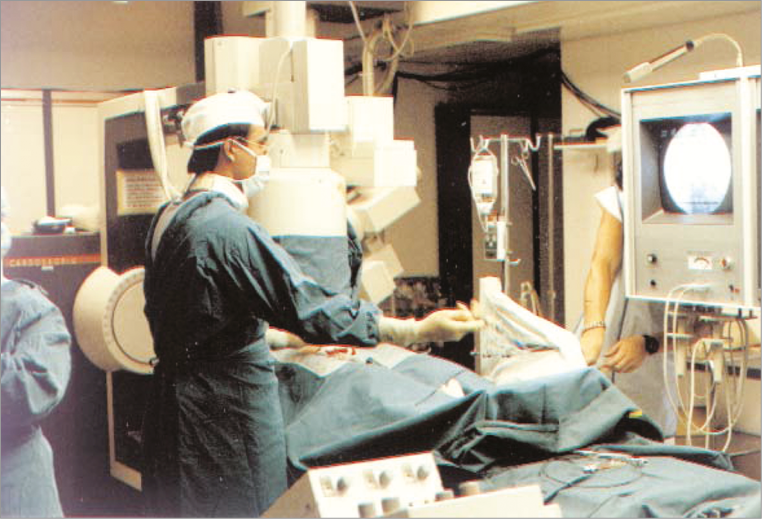
Figure 1. Andreas Grüntzig at work in the catheterisation laboratory where the first coronary angioplasty was performed on 16 September 1977. The resolution of the television monitor depicting the fluoroscopy was dismal and there was no option of replay or still frames of usable quality. Only the 35 mm cine films could be reliably interpreted but their processing took at least 30 minutes.
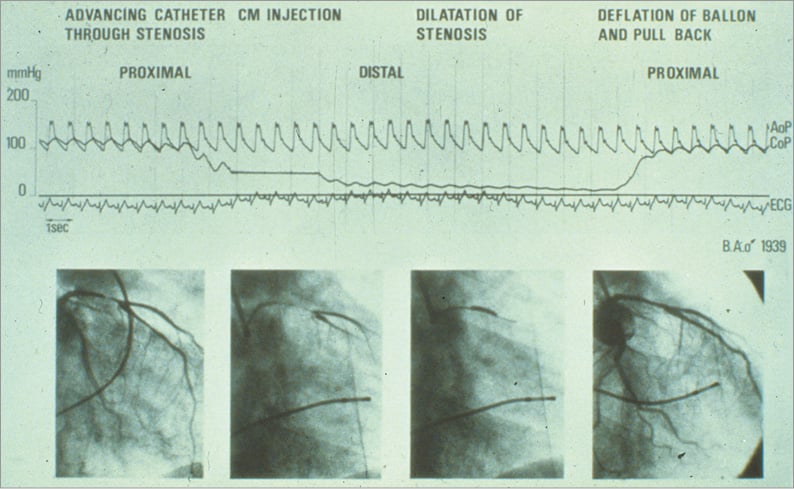
Figure 2. Documentation of pressures during the world’s first coronary angioplasty on 16 September 1977 at the University Hospital of Zurich in Switzerland. The top curve represents the aortic pressure at the tip of the guiding catheter in the coronary cusp (AoP). The bottom curve represents the pressure at the tip of the balloon catheter while in the coronary artery (CoP, damped because of the tiny lumen transmitting the pressure). The very low distal pressure during balloon occlusion (centre) documents the absence of collaterals. The distal pressure stayed practically level with the aortic pressure during balloon pullback at the end of the tracing. This testifies to a good haemodynamic result of angioplasty. The resolution of the cine frames depicted at the bottom is good as these are pictures of the processed cine film not available during the intervention. The initials and year of birth of the patient are depicted at centre right. CM: contrast medium; ECG: electrocardiogram

Figure 3. First balloon catheter for coronary angioplasty. Balloon catheter used for the first coronary angioplasty on 16 September 1977 at the University Hospital of Zurich, Switzerland. It was called Grüntzig Dilaca DG 20-30, referring to a length of 20 mm and a diameter of 3.0 mm. It was manufactured by Schneider Medintag, Zurich, Switzerland. There was a soft wire stub fixed to the tip (left side). The stub could be hand curved but not torqued from the other end of the catheter. It was therefore not steerable. The arrow points to the distal hole of the lumen used for pressure measurements. The marker to its right and the one at the proximal end of the balloon were visible on fluoroscopy. They were used for balloon placement.
Figure 4 shows the outcome of the first patient over almost four decades. Figure 5 shows the patient at the age of 38 years, shortly after his historic intervention (patient and doctor shared the same year of birth) and after a follow-up exercise stress test 39 years after his historic first and six months after his fourth and most recent coronary angioplasty intervention. Figure 6 illustrates that stress test.
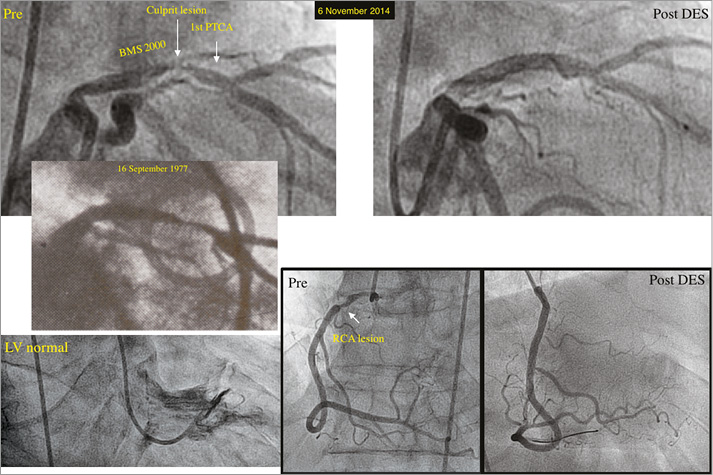
Figure 4. Aspect, result, and follow-up of the world’s first coronary angioplasty on 16 September 1977. The initial lesion is depicted as an insert in the middle of the left panel as present two days before and also at the beginning of the world’s first coronary angioplasty. Left top panel: bare metal stent (BMS), placed in 2000 proximal to the initial lesion (1st PTCA) and re-dilated a few months later for an in-stent restenosis, and recurrent (culprit) lesion dilated on 6 November 2014 with a drug-eluting stent (DES) with the result (right top panel). Left bottom panel: normal left ventricular (LV) function (end-systolic frame) on 6 November 2014. Right bottom panel: lesion in the proximal right coronary artery (RCA) before and after placing a DES on 6 November 2014. Post: after; pre: before

Figure 5. World’s first coronary angioplasty patient. The patient shortly after his historic first intervention (left panel) and six months after his fourth and most recent coronary angioplasty in front of a bust of Andreas Grüntzig (right panel). The insert at the bottom shows Andreas Grüntzig who was the same age as the patient (with permission of the patient).
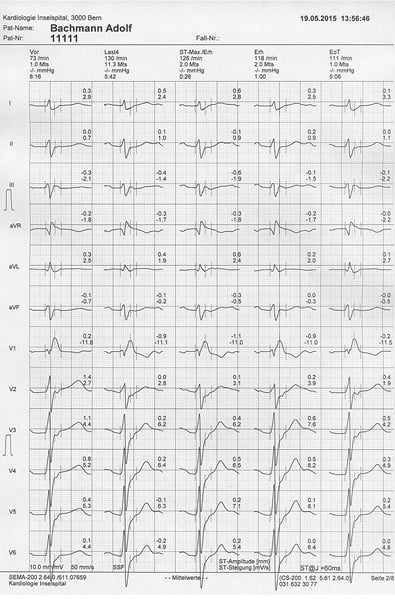
Figure 6. Exercise stress test in 2015, six months after the fourth and most recent coronary angioplasty of the world’s first patient. The right bundle branch block pre-existed for many years. Interestingly, it had already shown up for a few hours after the initial angioplasty in 1977. The patient had no symptoms and there were no signs of ischaemia at a maximal heart rate of 130 beats per minute and a maximal blood pressure of 205/90 mmHg. (with permission of the patient)
Inventing coronary angioplasty
Percutaneous transluminal coronary angioplasty (PTCA), as it was called before the name was changed to percutaneous coronary intervention (PCI), was not an overnight revelation to Grüntzig.
Several people were crucial to inventing balloon angioplasty and expanding it from the peripheral arteries to the coronary arteries. Charles Dotter was the first to apply catheter-based treatment to human vessels2. The Dotter procedure consisted of telescoping catheters of incremental diameters one over the other through the stenosis with the aim of dilating it. Unavoidably, the entry hole corresponded to the largest used outer diameter. Grüntzig in Zurich, Switzerland1, and Werner Porstmann in Berlin, East Germany3, simultaneously but independently experimented with balloon catheters to keep the entrance hole small and still reach the desired dilatation diameter. Porstmann failed with his idea to contain the outer diameter of a latex balloon by using it within a longitudinally sliced plastic catheter. Grüntzig succeeded with his idea to develop a sausage-shaped balloon that remained constant in form even under high pressure. Serendipity had led him across the street to the Technical University of Zurich, Switzerland, where he met a retired plastics expert (Heinrich Hopff) who suggested heat-treated polyvinyl chloride (PVC). Derivatives of PVC are still the material used for PCI balloons even today. While developing his balloon catheter, Grüntzig performed Dotter procedures (first on 15 December 1971) and continued to perfect them up to his first balloon angioplasty performed on a femoral artery on 12 February 1974. Figure 7 shows the team involved in Grüntzig’s peripheral balloon angioplasty programme during his transition to cardiology and before the first procedure in the coronary arteries.

Figure 7. Staff photo (1976) of the Division of Angiology. Peripheral balloon angioplasty team at the University Hospital of Zurich, Switzerland, in 1976 with the inventor Andreas Grüntzig in the centre, his tutor Alfred Bollinger third from right, his personal assistant Maria Schlumpf fourth from right, Felix Mahler, later the world’s first physician to perform renal artery balloon angioplasty, fifth from right, and the author first from right (courtesy of Maria Schlumpf).
Another person instrumental in Grüntzig’s successful development of PCI was Wilhelm Rutishauser. He lured Grüntzig away from angiology into cardiology, intuitively anticipating that something great was about to happen. Then there was Åke Senning, chief of cardiac surgery at Grüntzig’s institution, world-famous and an inventor in his own right. In spite of the fact that coronary artery bypass grafting (CABG) was not even 10 years old, he backed the competition emerging in the shape of interventional cardiology. So did his right-hand man, Marko Turina. He, together with Grüntzig, performed the dog experiments which form the basis of today’s PCI. Reassured by the good clinical outcome of most peripheral balloon angioplasties in spite of the initially not so appealing acute results with visible dissections, the anything but enthusiastic report of the pathologist examining the heart of the first dog undergoing PCI on 22 October 1975 (Figure 8)4 did not stop the two young and eager clinical scientists.
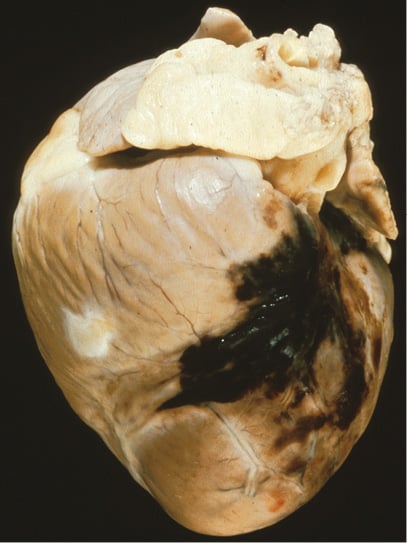
Figure 8. Aspect of the heart of the sacrificed dog after the first PCI experiment on 22 October 19754.
While Grüntzig indeed fabricated some of the balloons he used for clinical peripheral angioplasty at home, using standard glue and the kitchen stove, he relied on professional manufacturing for the coronary equipment which he obtained from Schneider Medintag, a small company producing medical equipment. Figure 9 shows the world’s only place to buy a coronary angioplasty balloon up to 1980. It was in the basement of an apartment house in a residential area of Zurich.

Figure 9. Schneider Medintag. Location of Schneider Medintag (circle), the world’s first and up to 1980 only producer of coronary balloon angioplasty equipment (Clausiusstrasse 62, Zurich, Switzerland).
It is interesting to note that even prodigies like Grüntzig with an infatuation for simplicity can be oblivious to the obvious for years. The initial inflation/deflation device (the misnomer inflation connotes air which was never used for balloon filling) is depicted in Figure 10. When PCI became a clinical routine, Grüntzig took the glass syringe out of this complicated apparatus and produced pressure by hand. It never occurred to him what is now trivial: using a disengageable thread to produce pressure easily by screwing. I cannot help but ascribe this to the fact that he rarely performed this tedious part of the procedure himself. When Grüntzig said we inflate for one minute at 6 bar, this meant for the assistant (e.g., me) that the small disc at the piston end of the glass syringe used at the time for this was going to sink painfully deeper and deeper into the palm while the pusher was breaking out in a sweat due to the effort of keeping up the pressure and the pain produced by it.
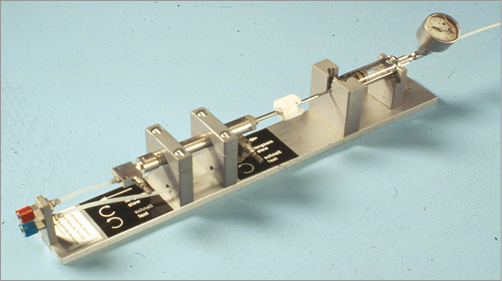
Figure 10. Inflation/deflation device for the initial coronary angioplasty procedures. A glass syringe filled with contrast medium was powered by air pressure and activated with a foot pedal. The syringe contained diluted contrast medium and featured a pressure gauge (top right corner).
FINDING THE FIRST PATIENT
It is hard to imagine these days that it took almost two years to find a patient with a single coronary artery lesion proximal enough to be reachable by the non-steerable PCI equipment produced for this purpose by Schneider Medintag according to Grüntzig’s instructions. At the time, coronary angiography was only performed in patients who had non-relenting angina pectoris for months, refractory to a combination of all available symptomatic drugs, such as nitrates, beta-blockers, and calcium antagonists. Moreover, they had to be current non-smokers and the upper age limit was closer to 60 than to 70 years. A patient who was free of angina after a myocardial infarction was not considered a candidate for an invasive workup. It comes as no surprise that patients finally undergoing coronary angiography were almost invariably found to have severe triple-vessel disease. After screening all local coronary angiograms for a suitable candidate to become the world’s first PCI patient for way over a year and also visiting centres abroad for the same purpose during that time, Grüntzig was confronted with the case of a 73-year-old man with intractable angina and heart failure due to recurrent myocardial infarctions. The patient had been turned down for cardiac surgery and had resided for weeks in the intensive care unit. Left ventricular function was poor and the descending aorta was occluded. On 22 March 1976 Grüntzig reluctantly tried to cannulate the stenosed left main coronary artery with the bulky guiding catheter (about 10 Fr) he had at the ready for the first patient. Having to use a brachial approach, selective coronary intubation failed and a balloon was not used. The patient succumbed to his untreated disease within the week.
In September 1977, I was taking care of a 38-year-old patient transferred from a peripheral hospital for recurrent resting and exertional angina for two weeks. An exercise test was performed in spite of the unstable situation, as was common at that time. It showed marked ST-segment elevation at peak exercise accompanied by chest pain. A radiologist performed coronary angiography on 14 September 1977 and described several lesions although there really was only one in the proximal left anterior descending coronary artery (Figure 4). Grüntzig was spending a week at a busy catheterisation laboratory in San Francisco, CA, USA, at that very moment looking for a suitable first patient for PCI there. It was the centre where he had successfully performed intraoperative catheter-based PCI together with fellow cardiologist Richard Myler and cardiac surgeon Elias Hanna on 9 May 1977. Upon his return on 15 September 1977, I presented Grüntzig with the cine film of this patient and he immediately wanted to be taken to the patient’s room to get his informed consent. The patient had been tentatively scheduled for CABG rather than just continuing medical therapy because he was so young (by coincidence the same age as Grüntzig) and so unstable. He shared a room with a man recovering from CABG. Sobered by this man’s tales of his suffering since surgery and displays of the scars on the chest and the legs, Grüntzig’s target patient was a pushover for his offer to try a much simpler technique on him, i.e., PCI. There was no written consent but I overheard Grüntzig mentioning to the patient that this had never been performed before in a patient and that there was a possibility that CABG would become necessary, perhaps even as an emergency procedure. This did not deter the patient, as CABG was his fate anyhow, short of this new procedure.
The rest of the story is unremarkable except for a few facts. First, the patient is still well and has never required CABG during the now almost 40-year follow-up (Figure 3-Figure 6). Second, Grüntzig kept a roller pump ready which he had used to perfuse the distal coronary artery bed during balloon inflation in the dog experiments. The dogs tended to produce ventricular fibrillation within a few seconds of balloon blockage. He did not use the pump for this first patient and he would not use it ever after. Third, poor fluoroscopy prompted Grüntzig to dilate the take-off of the first diagonal branch as well, which in fact was not stenosed and was not going to be stenosed for the subsequent four decades despite this inappropriate PCI in 1977. Fourth, the patient developed a right bundle branch block (RBB) during the procedure which spontaneously disappeared the following day. It was going to become permanent about 30 years later (Figure 6). The RBB and the recurring ST-segment elevation during an exercise stress test a few days after the procedure (this time without angina) were considered normal collateral phenomena of PCI. Now we know that they are not. Fifth, the original discharge letter (Figure 11) had Grüntzig only mentioned as a recipient of a copy. Hierarchy demanded that. It was much more rigid at the time in Swiss hospitals than it is nowadays. Sixth, acetylsalicylic acid was not yet known as a preventive therapy for coronary artery disease; vitamin K antagonists (Marcoumar in this case) were (Figure 11).


Figure 11. Original discharge letter of the world’s first coronary angioplasty case, signed by the author and another physician but not by Andreas Grüntzig.
Of note, the patient not only stopped smoking but he also discontinued all medications after a few months. In his opinion, medications taken chronically lose their effects. On the occasion of every one of the countless contacts I have had with him since, I have reminded him of the importance of preventive drugs but he had to pass his 70th birthday before he started to take a statin and a platelet inhibitor on a regular basis.
WHAT CAME AFTER THE FIRST CASE
If you think that this first successful case of PCI catapulted Grüntzig into the hall of fame of medicine, think again. Envy and jealousy rained on him at his workplace where he was mobbed by his superiors although the word mobbed had yet to be used in that sense. Foreign visitors sporadically but relentlessly flocking to his institution and trying to find Grüntzig’s office led to the moving of his office from the ground floor to the second basement. Having to give directions to visitors looking for Grüntzig’s office when parading through their department was obviously detrimental to the mental hygiene of some of his bosses. Pointing out where it had been moved to remedied that at least in part. Instead of garnering the fruit of having the world’s only doctor in the house who could offer PCI to mankind at the time, his superiors rationed the number of patients per week who could undergo PCI, first to one and then to two. As an admirer of Grüntzig, determined to help him and the procedure, I used the fact that I flew under the radar and was therefore more likely to go undetected when bending the rules and admitting more patients for PCI than allowed. These were almost exclusively distant referrals. Local patients were hard to find in the light of the then restrictive use of coronary angiography.
More than three years after the first case, Grüntzig caved in to the alluring offers from the USA, frustrated by the fact that he had accumulated only about 200 PCI patients in that time period due to poor logistics, made worse by unsupportive and unyielding superiors. He turned down Cleveland Clinic, Harvard, and Stanford among others in favour of Emory University of Atlanta, GA, USA. A generous grant of 100 million dollars to develop their medical facilities had just been offered to that institution by Coca Cola, headquartered in Atlanta. So, Emory could offer Grüntzig whatever he needed in terms of personnel, catheterisation laboratories, and equipment. Moreover, they made him a full professor of medicine, something the University of Zurich had adamantly refused and no German university had offered either. Cleveland Clinic could not offer that and Harvard and Stanford offered the title but a much more limited budget.
Among all the young doctors associated with cardiology at the University Hospital of Zurich, I was the only one interested in accompanying Grüntzig to the USA. The threat of losing support from the local medical establishment by teaming up with Grüntzig could have been a reason for that. Grüntzig appeared pleased with the idea that a local was interested in joining him in his new position. The fact apparently appealed to him that the people who had made his professional life so tough that he finally left Europe for the USA (a dubious place to live for somebody raised in the German Democratic Republic where Russian was taught but not English) would receive regular reports about how well he was doing.
In contrast to the old world in Europe, the new world in the USA was seduced by the potential of Grüntzig and his PCI and was eager to exploit it, irrespective of egos and traditions. Spencer King was ready to accept Grüntzig as a full partner in directing invasive cardiology at Emory in Atlanta, cognisant of the fact that the limelight would shine predominantly on Grüntzig, at least for a while. Grüntzig tried to make it easy for his new colleagues to accept him by bowing to and adopting many of their habits without necessarily “digging” them all. As an example, Figure 12 shows us getting acquainted with the local brew in Atlanta after our arrival there.

Figure 12. Andreas Grüntzig in the company of the author (left) on Stone Mountain in Atlanta (January 1981), shortly after emigrating there to develop coronary angioplasty further. Andreas Grüntzig insisted on drinking Coca Cola in Atlanta, according to the saying “when in Rome do as the Romans do”.
In the USA, virtually all his career wishes came true. Although he rapidly adapted to the American way of life, perfectly coached by his second (American) wife Margaret Ann Thornton, he occasionally mentioned during after-dinner chats that two things remained on his agenda. First, he would love to be awarded the Nobel Prize. He was actually proposed for it by several people but finally died much too early. Second, he would like to be called back as chief of cardiology to Zurich or a reputable university in his home country, Germany.
Teaching coronary angioplasty
Grüntzig was a gifted teacher but he also knew that unrestricted teaching devalues the teacher. He therefore taught thriftily if at all during routine work but generously when on stage. Figure 13 shows that he even carried the real equipment to the USA when he presented a first poster about coronary angioplasty at the annual meeting of the American Heart Association in Miami, FL, USA, in 1976. Although just a few people showed interest, he was eager to share all practical aspects of the technique with them.
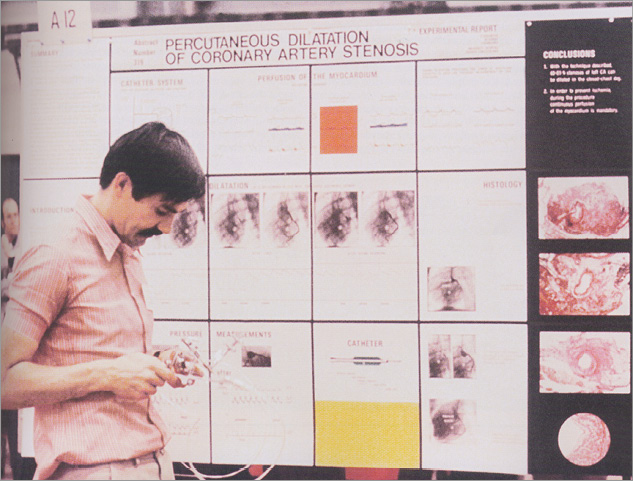
Figure 13. Andreas Grüntzig in front of a poster demonstrating percutaneous coronary angioplasty in dogs in Miami, Florida, USA, in 1976. He had the material ready to play with for interested visitors.
He also knew that he needed a few competent people in important places around the world to disseminate his procedure and he taught them everything he knew. Among them were Martin Kaltenbach in Frankfurt, Germany, and the two pioneers of PCI in the USA, Richard Myler in San Francisco and Simon Stertzer in New York (Figure 14). Myler later coined a saying about PCI that became legendary: “To become a fully accomplished interventional cardiologist you need to see one, do one, teach one, have one done, and do your mother”. It was based on fact in Myler’s case.

Figure 14. Optimal use of available equipment. Andreas Grüntzig (centre) showing his friends and American pioneers of coronary angioplasty, Richard Myler (front) and Simon Stertzer (back), that he not only cherished a simple way of treating coronary artery disease but also of commuting.
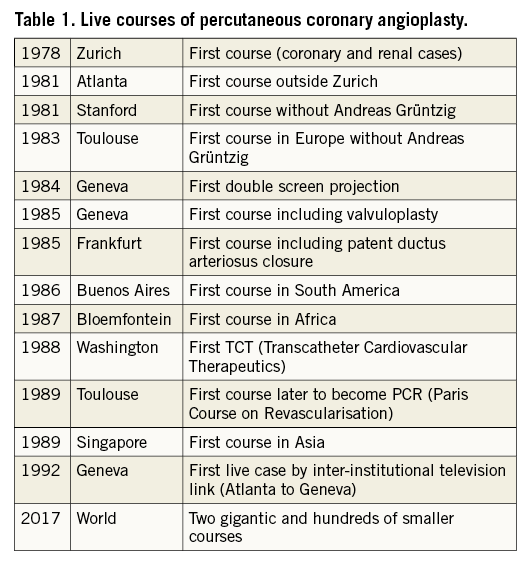
The next most important thing after PCI itself that Grüntzig contributed to modern cardiology was practical courses with live cases transmitted from the catheterisation laboratory to a lecture room. During these cases, Grüntzig liberally shared with his attending colleagues all he knew about the procedure. One of these secrets was how to adapt the guiding catheter to the individual requirements of the patient by custom shaping it or by cutting side holes (Figure 15). Table 1 shows some key points of the development of live courses. The first one in Zurich (7-10 August 1978) registered 28 participants, the second one (9-12 April 1979) 104 participants, the third one (2-5 January 1980) 171 participants, and the last one (3-7 August 1980) 212 participants (among them the icons of catheter-based cardiovascular therapy, Charles Dotter, Melvin Judkins, and Mason Sones). Tragically, they all died in 1985, the year of Grüntzig’s death. A census on the occasion of the last course in Zurich yielded a total of 819 PCI cases performed up to August 1980. Zurich maintained the lead (171 cases), followed by San Francisco (129) and New York (119).
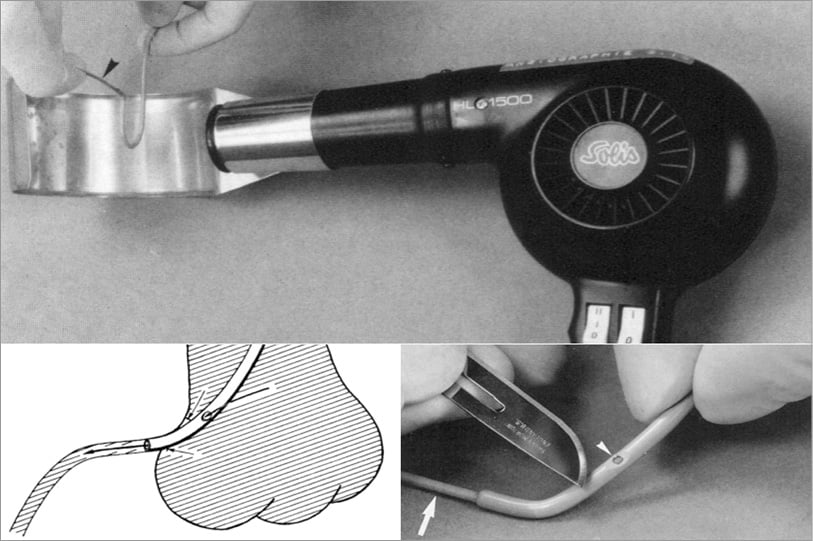
Figure 15. Adapting coronary guiding catheters. During the initial period while only a few guiding catheter shapes were available, it was frequently necessary to custom shape them during the procedure using an industrial hot air gun (top panel) or to create appropriate side holes for coronary perfusion while wedging the coronary ostia with the large guiding catheters (bottom panel).
In 1991, a paper analysed the results achieved during all live courses throughout the year and came to the conclusion that this teaching tool was ethically acceptable and that stenting provided an improvement over plain old balloon angioplasty but that other balloon alternatives or complements did not5. The debate about the value and limitations of live courses is still ongoing.
One of the most surprising messages which Grüntzig transmitted at these meetings was that, in spite of the delicacy of coronary arteries and the potentially life-threatening risk of occluding or perforating them, some aggressiveness and physical power was required and was appropriate to perform PCI successfully. For this he promoted the bimanual technique. The left hand of the operator manipulates the guiding catheter in contact with the groin (with the radial approach that would be the wrist) of the patient. The left thumb and forefinger control the guiding catheter, the left pinky rests on the skin. Meanwhile, the right thumb and forefinger advance and retract the balloon catheter, the Y-connector squeezed between the right pinky and ring finger. The Monorail technique was not yet available at the time and therefore the wire was controlled by a second person (Moving image 1). Nowadays, the wire needs to be controlled also by the right hand of the operator.
Collateral benefits of coronary angioplasty
Although PCI trailed 24 years behind the first catheter-based intervention in cardiology (cracking of a pulmonary valve stenosis with a wire-based instrument in a child)6, and four years behind a first wire recanalisation of an occluded coronary artery in acute myocardial infarction7, it turned the heads of clinicians, invasive cardiologists, and the industry. It also boosted the development of similar interventions. They pertain to valves, cardiac rhythm disorders, as well as congenital and other structural cardiac problems. Moreover, non-surgical procedures in other disciplines such as angiology, gastroenterology, gynaecology, neuroradiology, pneumology, radiology, and urology were initiated based on the success of PCI and still sail in its wake.
A look into the future by the man behind coronary angioplasty
Grüntzig was a sociable guy and liked to monkey around on occasions (Figure 16). Jokingly, he told me that someday a heart might be transplanted through a catheter. Cell therapy would have been a way to accomplish that, had it worked. He was more serious (but completely wrong) when he predicted that one day 15% of patients requiring coronary revascularisation would be treated with his technique. Little did he know that the development of facilities for invasive cardiology, mostly thanks to PCI, would allow early coronary angiography in millions of patients per year. This has enabled detection of the disease in its early stage, for which PCI was invented and in which it betters CABG. This is the main reason why, aided by technical developments, today Grüntzig’s prediction has been reversed and only 15% of patients requiring revascularisation still undergo CABG. Grüntzig did foresee coronary stenting but passed away before it happened. I regret never having asked him if he could foresee that heart valves were going to be replaced through a catheter someday. I reckon his answer would have been “why not?”.

Figure 16. Selfie, Grüntzig style. Andreas Grüntzig liked to party, which is illustrated in what may well be one of the first historically relevant selfies. To get this picture he laid his camera on the ground and invited everybody to look down on it until the self-timer went off.
Conflict of interest statement
The author has no conflicts of interest to declare.
Supplementary data
Moving image 1. Andreas Grüntzig explaining the bimanual technique. It is particularly helpful even today when it is necessary to intubate the coronary artery deeply with the guiding catheter to pass a tight lesion with the balloon. The voice in the background is that of Spencer King. The case was performed in Atlanta, GA, USA, in 1983.
Supplementary data
To read the full content of this article, please download the PDF.
Andreas Grüntzig explaining the bimanual technique. It is particularly helpful even today when it is necessary to intubate the coronary artery deeply with the guiding catheter to pass a tight lesion with the balloon. The voice in the background is that of Spencer King. The case was performed in Atlanta, GA, USA, in 1983.

