Catheter-based cardiovascular therapies: a seminal paradigm shift
In 1964, Charles Dotter demonstrated that the angiographic catheter could become an important surgical instrument. In 1977, Andreas Grüntzig added a balloon at the distal tip of a catheter to directly treat coronary stenosis. In 1978, Julio Palmaz had the idea to crimp a balloon-expandable stent onto the Grüntzig catheter to avert the limitations of balloon angioplasty. Ultimately, in 2002, Alain Cribier inserted a prosthesis inside a balloon-expandable frame to avoid surgical aortic valve replacement (SAVR). These disruptive approaches were first proposed for peripheral artery diseases and congenital heart diseases, then for coronary heart diseases and ultimately for structural heart diseases (Figure 1). We will focus here on the treatment of cardiovascular diseases, keeping in mind the fact that the same shift has occurred for non-cardiac vascular diseases. The accumulated experience with peripheral angioplasties was the necessary prerequisite that allowed Andreas Grüntzig to take the risk of dilating coronary arteries.
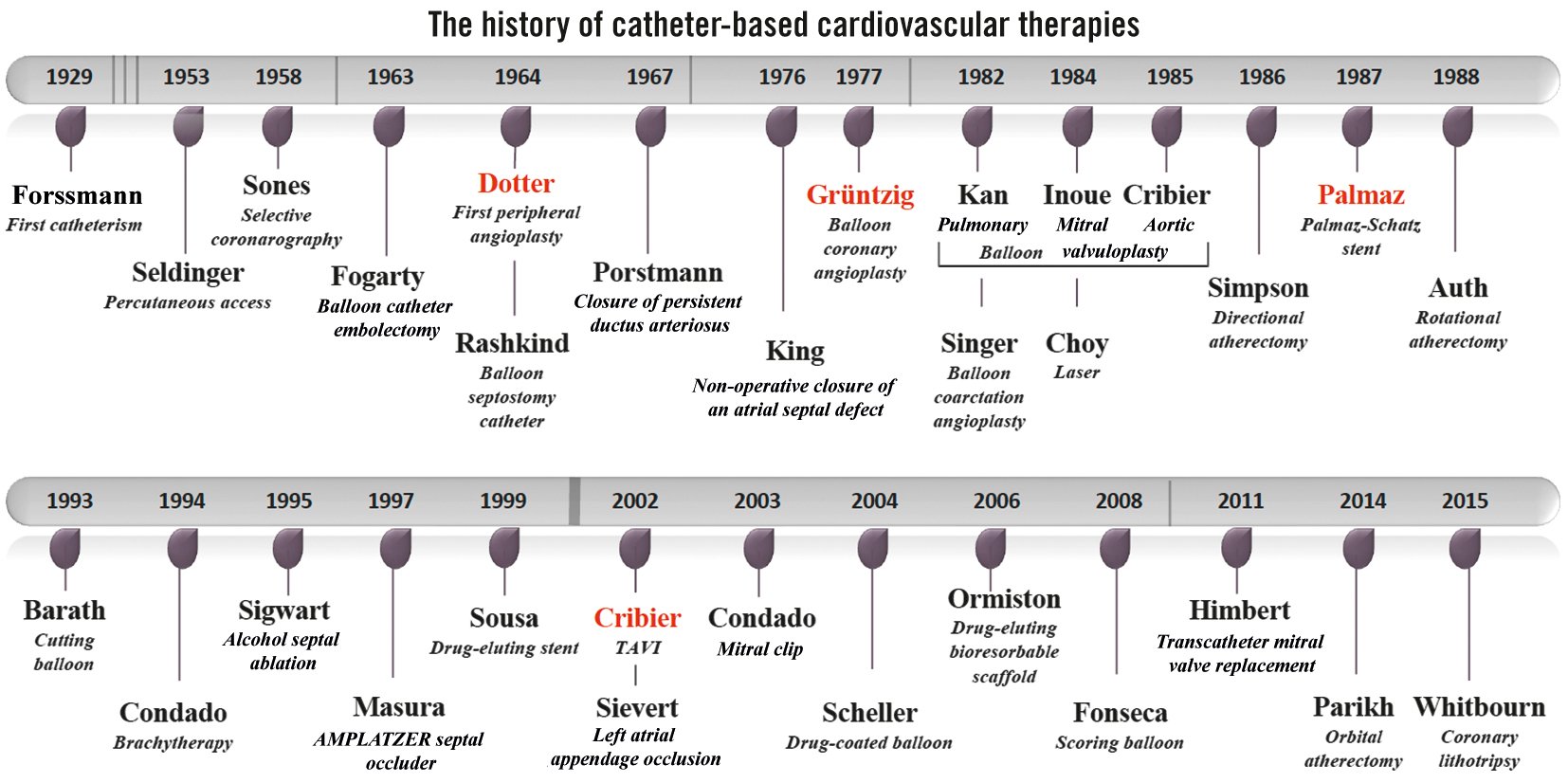
Figure 1. The history of catheter-based cardiovascular therapies. TAVI: transcatheter aortic valve implantation
Back to the origins of catheter-based therapies
The term “catheter-based interventional technique” was used for the first time in cardiology by Jeffrey Isner1 in 1987 regarding percutaneous balloon angioplasty of coarctation sites. A balloon was initially utilised therapeutically by Thomas Fogarty to remove clots in arteries in 1963, exactly 60 years ago. Then, in 1964, William Rashkind2 described the creation of atrial septal defects using a balloon-catheter technique as a palliative treatment for the transposition of the great vessels. Percutaneous transluminal balloon interventions, initially advocated by Andreas Grüntzig3 to treat coronary artery diseases, were used progressively in the 1980s in many other cardiovascular disorders: coarctation of the aorta (Singer in 1982) and more particularly stenoses of the heart valves: pulmonary valve (Kan in 1982), mitral valve (Inoue in 1984) or aortic valve (congenital [Lababidi in 1984] and elderly patients [Cribier in 1985]).
The term “device-based therapy” appeared much later, in 1998, in a study on transvenous implantable cardioverter-defibrillator systems to treat arrhythmic events4. Just 40 years earlier, in 1958, Ake Senning − who would help later Andreas Grüntzig perform his first angioplasties and provide the surgical stand-by − implanted the world’s first pacemaker. Countless devices have been used for the treatment of structural heart diseases, a term proposed by Martin Leon in 1999 at the Transcatheter Cardiovascular Therapeutics meeting, encompassing non-coronary cardiac diseases and developing interventional techniques.
Interventional transcatheter techniques originally involved congenital cardiac lesions. Werner Porstmann is credited with the first application of closing patent ductus arteriosus with a conical plug via a catheter system in 1967. A non-operative closure of an atrial septal defect was first performed in humans by Terry King in 1976 using a transvenous umbrella technique. Later on, Jozeph Masura used the AMPLATZER Septal Occluder (Abbott) − a self-expanding nitinol prosthesis − for the first time in 1997, which was also utilised later to occlude patent foramen ovale. Transcatheter techniques were further proposed for other procedures, such as alcohol septal ablation for hypertrophic obstructive cardiomyopathy by Ulrich Sigwart in 1995 and left atrial appendage occlusion by Horst Sievert in 2002 to avoid embolic cerebrovascular accidents in patients with non-valvular atrial fibrillation.
However, it was especially in the management of coronary heart diseases that the development of catheter-based therapies was the most remarkable. To secure coronary angioplasty, something needed to remain in the artery after dilatation. Jacques Puel implanted the first stent to treat restenosis in March 1986, and Ulrich Sigwart did the same for a bailout situation in June 1986. The advent of the stent revolutionised the practice of coronary angioplasty, eliminating the necessity of a surgical stand-by with the bare metal stent and permitting a drastic decrease in the rate of in-stent restenosis with the drug-eluting stent (DES). So far, drug-eluting bioresorbable scaffolds have not yet overtaken the best-in class DES.
Over time, new devices have facilitated coronary angioplasty: balloon technology has progressed, with cutting balloons, scoring balloons, drug-coated balloons and more recently, orbital atherectomy and lithotripsy, which facilitate the treatment of calcified coronary stenosis. Other techniques, like laser and brachytherapy, have gradually disappeared. Coronary total obstructions are now technically feasible in a large number of cases with the growth of new strategies and new tools. And, we are far from finished in our discovery of new transcatheter tools and devices to manage cardiovascular diseases, such as coronary angioplasty for complex high-risk indicated patients. As prophesied by Charles Dotter, a father to us all, “If a plumber can do it to pipes, we can do it to blood vessels.”
Transcatheter valve implantation: the ultimate achievement
If the first revolution in interventional cardiology occurred with coronary angioplasty, permitting the direct treatment of coronary stenosis without bypassing it, the second revolution happened with the first-in-human (FIH) transcatheter aortic valve implantation (TAVI) performed by Alain Cribier5 in 2002. Alain was the first to adapt balloon valvuloplasty to calcific aortic stenosis in 1985. Unfortunately, the spectre of restenosis had cast a shadow over this disruptive approach in elderly patients contraindicated for SAVR. The idea was to implant an expandable frame containing a prosthesis crimped on a balloon immediately after opening the stenotic valve with a balloon. And the brilliant vision of Alain was to use the calcifications of the native valve as an anchor to fix the prosthesis to, thus avoiding the possibility of embolisation.
As with any disruptive technology, Alain was faced with the opposition of the medical world who considered his idea totally unrealistic, fraught with major technical issues and that this procedure would never be approved by the U.S. Food and Drug Administration. It was concluded that it was the most stupid project ever heard of and that he had just to forget it⦠The same criticisms were made against Andreas by Hans Peter Krayenbühl, his hierarchical superior at the cantonal hospital of Zurich: “With Grüntzig’s procedure, patients will die!”, and against Julio Palmaz and Richard Schatz at the European Society of Cardiology meeting in 1990: “...it is unethical to put metal in human coronaries!” To overcome the opposition, Alain, like Andreas, bet on the fact that his intellectual honesty would sweep away any doubts about the relevance and the future of this percutaneous technique using catheters with balloons. Together they organised live demonstration courses in order to share and rapidly develop these innovative therapeutic approaches.
The PARTNER trials validated Alain’s vision with a significant benefit for TAVI in non-operative patients. For surgical patients, a significant benefit was observed for TAVI in low-risk patients with similar results in high-risk and intermediate-risk patients. Last but not least, minimalist TAVI has allowed us to simplify the procedure and to reduce the length of hospitalisation.
Concerning the mitral valve, the surgical edge-to-edge repair that permits the treatment of mitral regurgitation without valve replacement, proposed by Ottavio Alfieri in 1991, was adapted once again via the percutaneous catheter system by José Condado in 2003. Ultimately, the road traced by Alain Cribier for the aortic valve was taken over for the mitral valve. A novel treatment modality for failed mitral valve repair was proposed in 2010 by Arend de Wenger through the transapical approach, and Dominique Himbert6 performed the FIH percutaneous transcatheter mitral valve implantation via the transseptal approach in 2011. Here again, transcatheter tricuspid valve interventions (repair or replacement) are emerging as an alternative to surgery for severe tricuspid regurgitation.
Breaking new ground, Andreas Grüntzig had crossed the threshold into uncharted territory without imagining what the future of his technique could be. He said, “Whatever becomes of the method, I have left one mark on medicine. I have shown that man can work therapeutically within the coronary arteries in the face of an alert, comfortable patient.” The reality has surpassed all his dreams. Subject to the same criticisms and driven by the same enthusiasm, Julio Palmaz and Alain Cribier may be viewed as the two “spiritual sons” of Andreas.
Conflict of interest statement
The author has no conflicts of interest to declare.

