The history of interventional medicine is short, with an explosion of innovative treatments emerging at the end of the 20th century and dramatically expanding in the 21st.
For the newer generation of interventional specialists, with the armamentarium of these strategies at their fingertips, it is intellectually impossible to imagine what it was like treating cardiovascular disease less than 50 years ago. For those of us who were witness to that period and the developments that followed, it was another world.
The treatment of aortic stenosis (AS), the most prevalent valvular disease, stands out in this story – especially this year when we celebrate the 20th anniversary of the first successful implantation of an aortic stented valve in a human.
Importantly, it stands out not only for the scientific, technological and clinical achievements it represents but also as a testimony to the vision of the individuals – not just the specialists, but the first patients as well. Through their courage and insight they helped lay the foundations for what followed, reminding us that no matter how technical or device-oriented, the practice of medicine remains a human endeavour.
With an ageing population worldwide, the number of patients with AS is increasing each year. Their quality of life is severely curtailed and, as we know, if the disease is left untreated, death occurs in 80% of cases within three years after the onset of symptoms. Before the advent of effective interventional techniques, the only option we had as cardiologists to treat patients with severe symptomatic AS was open-heart surgery, but many of these patients were deemed too frail to undergo or were incapable of surviving the surgery if they did. With no other medical treatment available, these inoperable patients were, in the short term, simply condemned.
This was the context – the glaring and unmet clinical need – that we all faced in 1985.
But before looking at the development of transcatheter aortic valve implantation (TAVI), here is a brief review of the history of treating “structural heart disease”. The term, first coined by Martin Leon at the TCT meeting in 1999, was quickly adopted. Covering all non-coronary heart diseases along with their dedicated interventional techniques, the field saw rapid growth from the transcatheter treatment of congenital valve disease: from pulmonic stenosis in 19791 and aortic valvular stenosis in 19832 to mitral valvuloplasty3 and aortic valvuloplasty in adults in 1984 and 1985, respectively4, followed by pulmonic valve replacement in degenerated conduits in the 2000s5.
The successful percutaneous treatment of aortic valve stenosis in inoperable patients remained a challenge. In September 1985, faced with a 72-year-old female patient with calcified AS, recurrent syncope and imminent death, Professor Cribier and his Rouen University Hospital team adapted an existing technology from the field of congenital valve disease. Under local anaesthesia, a pulmonary balloon was employed to dilate the patient’s aortic valve using a percutaneous transcatheter femoral approach. This first-in-human balloon aortic valvuloplasty led to an immediate clinical and haemodynamic improvement, followed by relief of symptoms and a return to normal life for the patient. The results were published soon afterwards in The Lancet4, and the cardiology community worldwide quickly embraced balloon aortic valvuloplasty (BAV). This technique was enthusiastically adopted as the only life-saving procedure at the time for treating inoperable patients. However, after several years it became evident that balloon dilatation was not a long-lasting solution due to restenosis, with a return of symptoms in 80% of patients at one-year follow-up. The fact that restenosis was found produced deep disappointment in the medical community, especially after the excitement generated by the earlier positive results.
Thus, at the very beginning of the 21st century, though certain approaches had been tried and others were being researched, the specific challenges in finding an effective percutaneous treatment for AS remained elusive, with few treatment options for inoperable individuals. The reaction of the cardiology community to the failure of BAV was merciless and created a tidal wave of criticism concerning the work of the Rouen team. This criticism only served to reinforce their resolve to find a viable solution to deal with the restenosis that undermined BAV.
The concept of implanting a dedicated stented valve for calcified AS was born out of this challenge. Having observed that the circular shape of the balloon could expand the calcified aortic valve, the Rouen team believed that it was possible to open the orifice despite the calcification. The idea that developed was to maintain the initial positive results of the expansion by implanting a stent with a valve inside using the diseased native calcified valve of these inoperable patients itself as an anchor rather than removing it. This would be done under conscious sedation on a beating heart, employing the catheter-based techniques already in use.
This concept created other issues besides the nature of the diseased native valve itself; critical questions arose concerning the immediate proximity of essential anatomical structures. Intensive, in-depth research with autopsies and in vitro experimentation were undertaken to determine and validate the correct dimensions of the stented valve to ensure there would not be damage to the surrounding structures, such as to the coronary arteries, and to the mitral valve: a key step in guaranteeing the feasibility and safety of the concept.
During this same period in Denmark, Dr Henning Andersen developed a dedicated stented valve for treating aortic valve regurgitation and other vascular diseases, but not for aortic calcified valve stenosis. However, he did not follow up on his initial experimentation6.
The adventure of perfecting the stented valve was only beginning. For over five years, the Rouen team failed to convince biomedical manufacturers to develop a prototype, the project being considered as totally unrealistic. At the same time, a new technique was developed in Rouen – mitral commissurotomy using a metallic dilator – for the treatment of mitral valve stenosis, which was subsequently widely used in developing countries.
It was in 1994 that development of this innovative concept started to accelerate when Professor Cribier met engineers from Johnson & Johnson, Stan Rabinovich and Stan Rowe, who expressed interest in the project. In 1999, with Martin Leon, they created the start-up “Percutaneous Valve Technology”. With the help of investors, a prototype was designed by the Aran R&D company in Israel. Using the sheep model, extensive studies and evaluations of this prototype proved the concept’s feasibility.
The first-in-man implantation of this revolutionary device was performed as a last resort option in a 57-year-old patient in Rouen, France on 16th April 2002 (Figure 1), and was subsequently reported in Circulation7. The success of the new technique was dramatic, and the start-up was acquired in 2004 by Edwards Lifesciences, leading to an evolution in the technology that continues to this day.
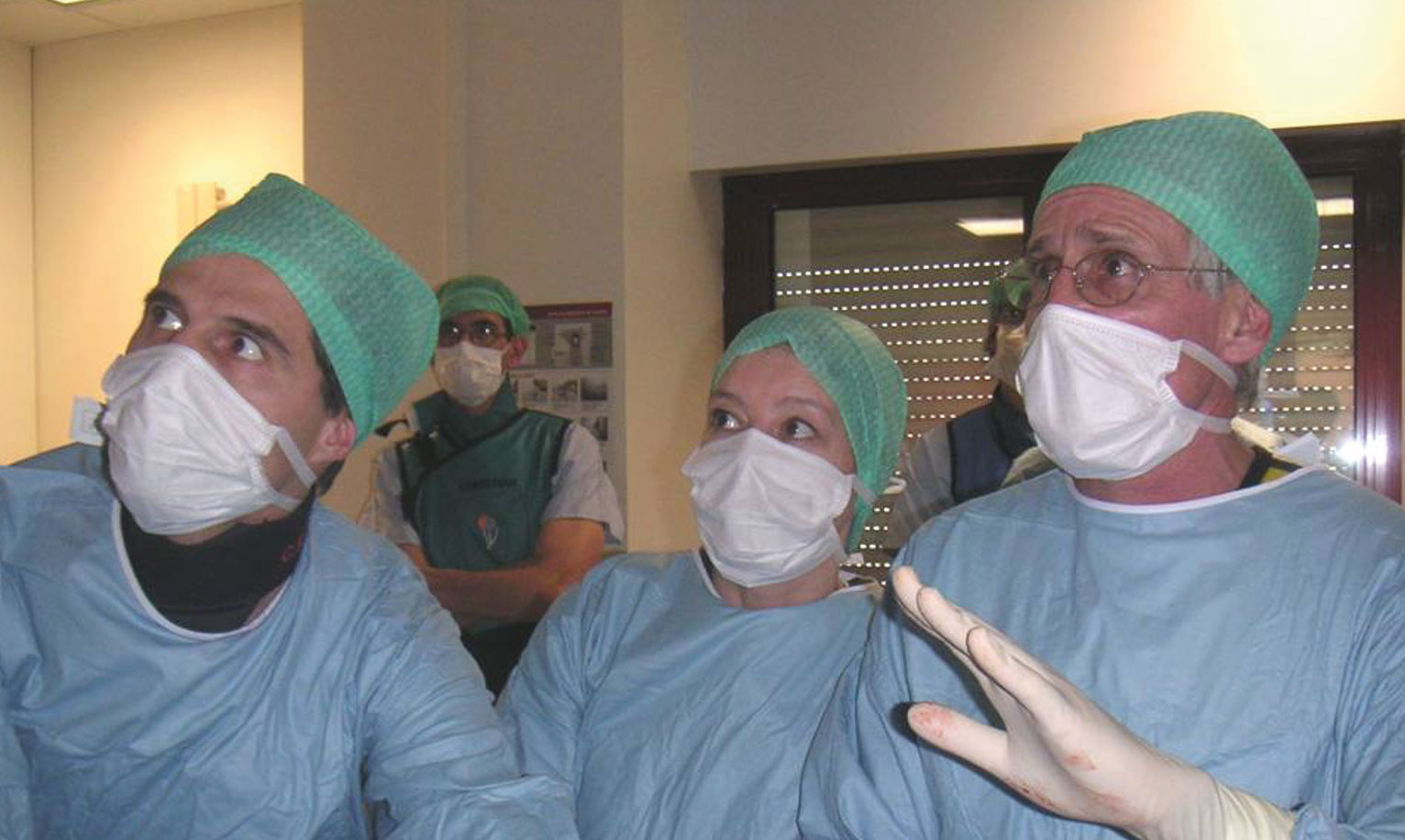
Figure 1. First-in-man TAVI performed at at the Rouen University Hospital, Rouen, France, 16 April 2002: From left to right: Christophe Tron, Hélène Eltchaninoff, Alain Cribier
This has from the start been a development based on scientific rigor and clinical excellence which fulfil the most stringent requirements of medicine. Since that first successful TAVI 20 years ago, TAVI has been at the centre of an impressive series of scientific evaluations, randomised trials and international registries. This work has resulted in many thousands of peer-reviewed publications where trials have demonstrated time and time again the non-inferiority, and even superiority, of the transcatheter approach to cardiac surgical replacement. Registries, such as FRANCE8, FRANCE-29 and FRANCE-TAVI10, have played an essential role in fully grasping the extent to which TAVI has advanced and become part of our practice, while offering the critical data necessary for a probing meta-analysis that provides a foundation for the future evolution of the technique. It is through this careful process of evidence-based medicine that a treatment, originally limited to “compassionate” use in high surgical risk patients, has been enlarged by the American and European guidelines to include patients at intermediate risk and finally, in 2021, to include low surgical risk patients as well. In numerous countries, the number of TAVI procedures now exceeds the number of surgical valve replacements1112.
The number of TAVIs has increased by 10% per year for several reasons:
– A continuous improvement in technology which has increased the safety of the procedure, in part by reducing the complication rate.
– A growing experience on the part of the individual operators as well as the Heart Teams with an increase in the number of specialised centres practicing TAVI. When combined, experience and accessibility have made TAVI an easy procedural choice where in the past cardiac surgery would have been the only option.
– The continued expansion of indications to low-risk patients will occur as durability of the implanted valves is demonstrated. This has encouraged research on different types of valves less prone to deterioration.
– The continued and future expansion of TAVI to other indications such as valve-in-valve procedures (e.g., treating future TAVI dysfunction) or the treatment of moderate AS in patients with heart failure, etc.
– Also, importantly, the increase in TAVI procedures and the vital competition between devices has led to an overall decrease in the cost of TAVI which is of particular interest for lower-income and developing countries.
– And of course, the outstanding combination of excellent clinical results and the simplicity of the procedure for the patients themselves: no sternotomy, the use of local anaesthesia, a short hospital stay and, most importantly, a rapid return to normal life.
This steady expansion is, like the development of PCI (angioplasty and stenting), an excellent example of successful translational research, moving findings from concept to bench, bench to bedside, feasibility trials to larger clinical registries, and evidence-based trials to everyday practice. The constructive dialogue that continues between multidisciplinary physicians and engineers has been central to the evolution of TAVI. However, it is of interest that TAVI, unlike PCI, began with the most untreatable of patients and only now is being used in lower-risk groups.
In the advances in the treatment for valve disease TAVI played – and continues to play – a seminal role. It stands as a lightning rod and catalyst – an impetus for research and a creative idea spurring on device and clinical innovation.
Looking back over the last 20 years since that first TAVI, we are astonished by the impact this procedure has had. TAVI crystallised the study of structural heart disease, inspiring a younger generation of interventionalists and scientists. It remains an innovative and disruptive technology influencing many of the ways that medicine is practised today. In cardiology alone, it paved the way for percutaneous transcatheter treatment of the mitral and tricuspid valves, has led to far-reaching developments in cardiac imaging and has been instrumental in bringing together specialists in the Heart Team concept.
To all those who believe that we as cardiologists can make a difference in our commitment to ideas, TAVI stands as an example of what such an idea can produce. TAVI has made its mark on all of us, in more than 1,500,000 patients who have been successfully treated today, and in the seeds it has planted in the way we practice medicine and advance our research and clinical practice.
After twenty years, the future stories of TAVI are still being written.
Acknowledgements
We would like to thank Sheldon Heitner for his help in preparing this manuscript.
Funding
H. Eltchaninoff and M. Gilard have received a grant by the French Government, managed by the National Research Agency (ANR) under the program “Investissements d’avenir” with the reference ANR-16-RHUS-0003. H. Eltchaninoff is also supported by a grant from the GCS G4 as part of the FHU-CARNAVAL, labelled by AVIESAN.
Conflict of interest statement
A. Cribier is a consultant for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

