
It has been a great privilege for all cardiologists who, like me, started their experience of cardiac catheterisation in the early 1970s, to follow the fascinating evolution of the technology over the past 40 years. For all of us, the crucial milestone has been the invention of percutaneous transluminal balloon angioplasty by Andreas Grüntzig, and the first coronary angioplasty performed by him in September 1977, of which we are celebrating the 40th anniversary. We can be profoundly indebted to him, not only for having introduced such a revolutionary creative approach for the treatment of coronary artery disease, initiating at the same time the specialty of “interventional cardiology”, but also for having been such a great source of inspiration for many others. If he was still among us, Andreas Grüntzig would probably be amazed and proud to see that his invention catalysed the incredible expansion of transcatheter interventions in cardiology to a wide range of congenital and acquired heart diseases, far beyond angioplasty and stent implantation.
In the field of “structural heart diseases”, a term introduced by Martin Leon at the Transcatheter Cardiovascular Therapeutics meeting of 1999 to cover all non-coronary heart diseases and their dedicated interventional techniques, valve replacement and repair have been the fastest developing areas. Following in the footsteps of transcatheter treatment of congenital pulmonic (1979)1 and aortic valvular stenosis (1983)2, mitral valvuloplasty (1984)3 and aortic valvuloplasty in adults (1985)4, then pulmonic valve replacement in degenerated conduits (2000)5, the technique of transcatheter aortic valve implantation (TAVI)6 emerged in 2002 to alter the landscape of cardiovascular medicine profoundly. In its turn, TAVI became a strong source of inspiration for the development of new interventional techniques aimed at treating other valvular diseases, such as mitral valve repair and, more recently, mitral valve replacement. With the increased prevalence of valvular diseases with age, over the next decade one can expect a 30% growth of interventional cardiology in this field7.
Successful medical device development is rarely easy but few blockbuster technologies, such as TAVI, have faced so many challenges and achieved such great success. Widely accepted by the medical community, TAVI has been performed in about 300,000 patients, with an annual compound growth rate of 40%, and is now available in 65 countries around the world. There are few examples of clinical fields in medicine that can match the rapid and careful evolution of TAVI. Similar to the development of PCI (angioplasty and stenting), the development of TAVI can be viewed as a fascinating example of successful translational research, with findings moving from concept to bench, bench to bedside, feasibility trials to larger clinical registries, and evidence-based trials to everyday practice. The outstanding interaction between multidisciplinary physicians and engineers has been the key to success.
At the beginning, a common point of these two major innovations, TAVI and PCI, was the fierce opposition of experts, the majority being cardiothoracic surgeons, all staunch detractors of these “totally unrealistic and stupid ideas” that will “never work”. It took a lot of self-conviction, courage and perseverance to knock down the wall of such opposition. On the other hand, TAVI and PCI differ in terms of the drastically different clinical pathway imposed by health authorities for clinical evaluation. TAVI, this brand-new technology based on a highly challenging concept, had to be tried first on dying patients, those patients “with one foot on a banana peel, the other foot in the grave”, to expand cautiously to inoperable or high surgical risk patients and, eventually, after 15 years, to lower-risk patients. Such a terrible pathway could easily have killed the technique in its early stages. In contrast, PCI followed the exact inverse and more acceptable pathway, starting with “simple cases” (proximal LAD lesions), expanding progressively to complex cases over a similar period. In this regard, the immense success of TAVI is particularly meaningful.
Moving from balloon aortic valvuloplasty to the concept of TAVI
In 1994, the TAVI innovator’s crazy dream was incredibly challenging: “implanting a balloon-expandable valve within the diseased calcific native valve (i.e., without cutting it out), on the beating heart, using regular percutaneous catheter-based techniques, under conscious sedation, in inoperable AS patients”. Obviously, more than 20 years later, the reality clearly surpasses the dream.
Moving from concept to human implantation was the most fascinating phase of the adventure. For the pioneer, the starting point of TAVI emerged in the early 1990s with the obsession of finding a solution to early restenosis after balloon aortic valvuloplasty (BAV). Pioneered by us since 1985, BAV underwent an extraordinary world expansion. The goal was to address an important unmet clinical need for a common disease by providing a new, less invasive therapeutic option for elderly symptomatic aortic stenosis (AS) patients considered to be inoperable, not only because of comorbidities increasing the risk of surgical aortic valve replacement (SAVR), but also because of age >75 years which was, in the 1980s, a customary contraindication to SAVR. These patients were threatened with death within a couple of years without aortic valve replacement. After the first-in-man case performed in Rouen in September 19856 (Figure 1), followed by many live-case teaching seminars (Figure 2) and much worldwide on-site proctoring, BAV was evaluated in multiple national and international European and US registries, and reported in more than 1,000 peer-reviewed articles. However, whereas midterm improvement of quality of life was regularly observed, recurrence at short term was an important limitation, leading to the progressive decline of BAV8. Nevertheless, the procedure nowadays remains a palliative therapy for limited subsets of elderly or severely ill patients with no surgical or TAVI option, and BAV is also integrated into many TAVI procedures.
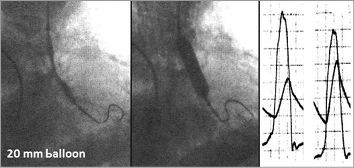
Figure 1. First-in man balloon aortic valvuloplasty, Rouen, September 1985. Haemodynamic result after 20 mm balloon inflation.

Figure 2. First International Seminar on Balloon Aortic Valvuloplasty, Rouen, February 1999.
Several catheter-based systems for the treatment of valvular disease had been investigated since the 1960s, all projects remaining at the experimental stage9-12. A new era of investigations started with the development of endovascular stents, introducing the concept of a balloon-expandable valvular prosthesis. Andersen et al13 reported their work in a porcine model in which they evaluated a transluminal stented heart valve aimed at treating various cardiovascular diseases. Here again, there was no human application. In 2000, Bonhoeffer et al, using a valve from a bovine jugular vein mounted within an expandable stent, reported the feasibility of delivering the device inside the native pulmonary valve of lambs14 and thereafter were able to perform the first successful human percutaneous replacement of a pulmonary valve in a right ventricle to pulmonary artery prosthetic conduit with valve dysfunction5.
The idea of implanting a balloon-expandable stented valve within the diseased native valve came from the observation that, during BAV, all calcified valves could be circularly opened by high-pressure balloon inflation. The highly original idea was to keep the disease and use it to anchor the stent frame! At first sight, stenting aortic stenosis posed insurmountable problems, not only because of the rocky nature of the diseased native valve, but also because of the immediate proximity of essential anatomical structures, coronary arteries and mitral valves. This unlikely concept was however remarkably validated in 1994 in a landmark cadaver study that we conducted in Rouen on fresh AS specimens (Figure 3). All 12 calcific aortic valves could be circularly opened with balloon-expandable peripheral Palmaz stents, 23 mm in diameter. The study also established the optimal stent height (14 to 16 mm) to avoid impinging on the coronary ostia, the anterior mitral valve leaflet and the upper interventricular septum containing the bundle of His. Furthermore, removing the stent from the fibrotic calcific valve required a pair of pliers, thus making the underlying risk of device dislodgement and embolisation after implantation unlikely. The valvular structure and the way of attaching it to the frame were imagined and described. With the help of our cardiac surgeon, J.P. Bessou, a model of the device was handmade, the diameter of which, after crimping over a BAV balloon catheter, did not exceed 8 mm, making the retrograde transfemoral approach that we had in mind conceivable (Figure 4). However, over a period of four years, the project was constantly turned down by all biomedical companies. All experts considered the idea technically impossible and clinically irrelevant, pointing out, besides major engineering issues, a long list of life-threatening complications such as coronary obstruction, mitral and aortic regurgitation, endocarditis, stroke, device embolisation, etc. However, eventually, perseverance won out! In 1996, I met the right people at the right time, Stan Rabinovich and Stanton Rowe, two engineers from Johnson & Johnson (J&J), who did support the idea! With their help, an agreement was signed to develop the valve. The company filed my patents, but a year and a half later nothing had been done. The project was then brought to Martin Leon with whom Rabinovich and Rowe had been working at J&J on coronary stent trials. His feedback about the idea of a transcatheter aortic valve was very positive and he became a steadfast supporter of the project. This was the start of a great commitment, cooperation and friendship between two physicians and two engineers (Figure 4).
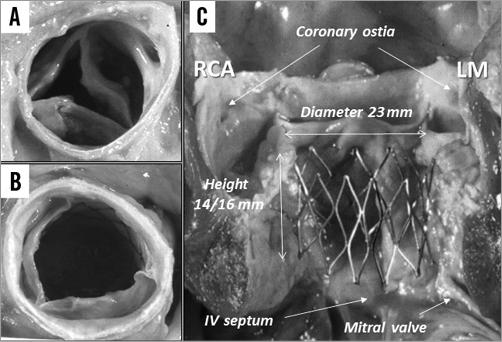
Figure 3. Autopsy study on AS (1994). A) Effect of 23 mm balloon inflation. B) Circular valve opening with 23 mm Palmaz stent. C) Longitudinal view: optimal stent dimensions to preserve the surrounding structures.
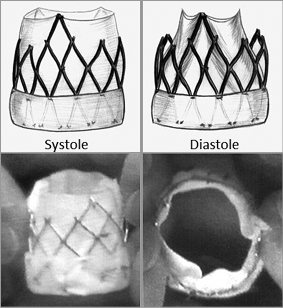
Figure 4. First drawing of the transcatheter valve and corresponding handmade model, Rouen, 1995.
The percutaneous valve technology era: moving to first-in-man and feasibility trials
In 1999, a start-up company was founded by the four of us (Percutaneous Valve Technologies [PVT], Fort Lee, NJ, USA), with the goal of developing the percutaneous valve. One of the first challenges was to find an engineering partner that would help to develop the device. A company in Israel was identified, ARAN R&D; at the time they wished to invest in medical devices (Figure 5). They had just hired an outstanding engineer, Assaf Bash, from Medtronic, with whom we could share the philosophy of the transcatheter heart valve and establish the numerous issues of preclinical engineering (Figure 6). Various prototypes of transcatheter valve were rapidly created, starting with polymeric valves, moving later to tissue valves. Each model was intensively evaluated in the laboratory, using new tools for a new technology (Figure 7), and on the animal model. With my collaborator, Helene Eltchaninoff, extensive acute experimental studies were conducted on the sheep model, a challenging model whose particular aortic anatomy and healthy valves left unanswered the issue of the safety of TAVI in human calcific AS. However, this difficult experimental work led to the staged improvement of valves, delivery systems, crimping devices, and techniques of implantation. An original and crucial experimental approach was eventually created to get a five-month implant in the descending aorta15 prior to the first-in-man. The final device was a trileaflet bovine pericardial valve incorporated within a stainless steel stent the expanded diameter of which was 23 mm (Figure 8). The system was compatible with a large 24 Fr Cook introducer sheath (Cook Medical, Bloomington, IN, USA).

Figure 5. The outstanding ARAN R&D team, Caesarea, Israel.
Figure 6. The PVT team with Assaf Bash. From left, Stanton Rowe, Alain Cribier, Martin Leon, Assaf Bash, Stan Rabinovich.
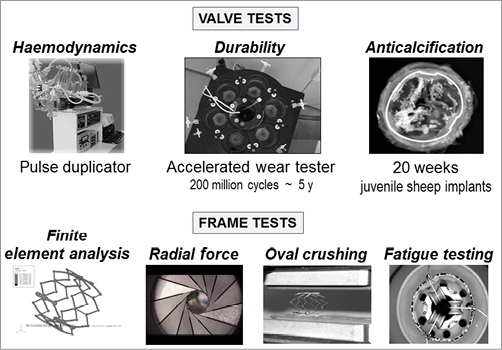
Figure 7. Valve and frame in vitro testing: new tools created for a new technology.
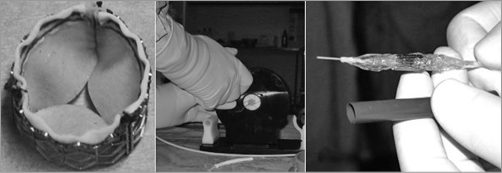
Figure 8. The finalised PVT percutaneous heart valve system. A) The PVT transcatheter heart valve. B) Original crimping device. C) Valve crimped over a 23 mm Numed balloon, compatible with a 24 Fr Cook introducer.
The first in-human implantation performed in Rouen on 16 April 2002 in a 57-year-old desperately ill man in cardiogenic shock, with critical AS, inoperable because of multiple comorbidities, will remain a memorable day. He presented with most current contraindications of TAVI: ejection fraction 12%, intraventricular thrombus, and subacute ischaemia of the leg due to a failed aorto-bifemoral graft. This patient had been turned down by three surgical teams. After a failed emergent BAV, TAVI appeared to us the only life-saving option for this young patient. He and his relatives were ready to accept any first-in-human experimental treatment. To make the decision more complex, whereas the procedure had always been built around a transfemoral retrograde approach, TAVI required to be carried out using a transseptal route, which entailed many questions about the procedural feasibility. Even though our team had good experience of transseptal plain BAV, and considerable experience of transseptal catheterisation for mitral commissurotomy, implanting an aortic valve device was another story, requiring the invention of each procedural step. There were a lot of exchanges among PVT’s partners about the risky situation and, at the end of the day, they took the decision of trying it for ethical reasons. Despite the dreadful clinical setting, the procedure was carried out successfully (Figure 9, Figure 10) and led to a spectacular immediate improvement in the patient. In less than one hour, the patient’s face turned from grey to pink. Haemodynamic and echocardiographic results were incredibly improved, with no significant aortic regurgitation, no impairment of the coronary arteries and mitral valve, and a circular opening of the frame, a great translation of our previous autopsy findings. The patient was sitting up the next morning. No words can express the emotion felt by the whole team, or the incredible stupefaction of the medical community at the report of this first case6. It was however impossible at that time to predict the future of this revolutionary medical therapy.
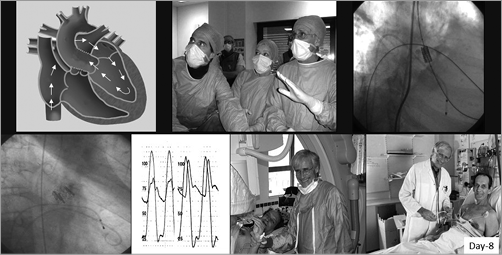
Figure 9. First-in-man TAVI, 16 April 2002. Transseptal route, phases of implantation, and patient clinical improvement.
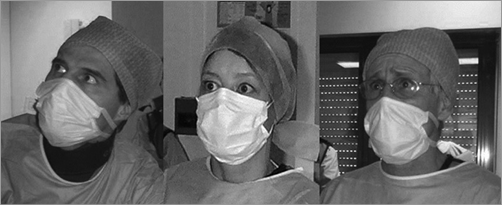
Figure 10. The unforgettable stressful moment of truth at the time of valve deployment, 16 April 2002. From the left: C. Tron, H. Eltchaninoff, A. Cribier.
From 2002 to 2005, two feasibility studies including 36 severely ill patients were conducted in Rouen, after approval by the French health regulatory agencies for compassionate use only (I-REVIVE and RECAST trials)14,15. At the time of I-REVIVE, the device was modified by using an equine pericardial valve. The retrograde approach was tested in seven patients but failed in three due to the lack of any specific delivery system. The feasibility of TAVI was clearly confirmed with a 75% procedural success rate. Of note, rapid ventricular pacing during valve deployment was introduced by us in this trial, which remains crucial today for accurate balloon-expandable valve positioning. An incidence of 44% of grade 2 and 11% of grade 3 aortic regurgitation was noted, obviously related to the unique 23 mm valve size available, and the incidence of MACCE at 30 days was 26%. If several of these critically ill patients died of their comorbidities within weeks or months, it is quite remarkable that several did survive several years (as much as four, five, or even 6.5 years) with no device failure and a return to normal life. The extension of the protocol to other centres in the USA and Europe clearly showed the limitation of the transseptal approach in less experienced hands. In 2005 the total number of TAVI had reached 100 cases, and further expansion of TAVI clearly deserved technical and procedural improvements.
The Edwards Lifesciences era: TAVI takes flight and expands across the world
In 2003, several major US companies which had become investors in PVT started to believe strongly in the future of this disruptive technology and started thinking about acquiring PVT. In 2004, PVT was eventually acquired by Edwards Lifesciences (Irvine, CA, USA), the leading heart valve company. With this acquisition, TAVI entered a new era. The SAPIEN valve offered all the physical characteristics of the Carpentier-Edwards valve and was available in two sizes, 23 and 26 mm. New delivery systems were developed, conceived for the transfemoral retrograde and transapical access routes, evaluated by J. Webb et al in Vancouver16 and T. Walther et al in Leipzig17, respectively. The launch of the transapical approach led to improving the surgeon’s vision concerning TAVI (Figure 11). Multiple European, Canadian and US feasibility studies were undertaken with the SAPIEN valve, whereas a concurrent valve, the self-expanding CoreValve (Medtronic, Dublin, Ireland), had been on the rise in Europe since 200418. After European certification of the two devices in 2007, the evidence-based validation of TAVI was achieved in the USA with the Edwards valve in a broad randomised FDA-driven pivotal study, the Placement of Aortic Transcatheter Valves (PARTNER) trial. This study established TAVI as a standard of care in severe AS patients without surgical options and as an alternative to surgery in high-risk patients. The new culture of a disease-state model requiring multidisciplinary physician collaboration in a “Heart Valve Team” was also beautifully addressed in this trial. The results published in four issues of the New England Journal of Medicine19-22 led the FDA to approve TAVI in 2011 and 2012 in these two respective indications. In 2014, the FDA granted approval of the CoreValve after reviewing the results of the CoreValve US Pivotal Trial in high-risk patients, showing the superiority of TAVI over surgery in terms of mortality at one year23.
Figure 11. First transapical case, Leipzig, Germany, 2014. The devil enters the operating room. From the left, Michael Mack, Alain Cribier, Friedrich Mohr.
The expansion of TAVI was thereafter considerable, thanks to staged refinements in valves and delivery systems, procedural techniques, patient selection, new multimodality imaging technologies and biomedical engineering of accessory devices, leading to dramatically improved clinical outcomes. Broad national controlled registries on thousands of patients consolidated the increasing place of TAVI in high-risk patients24-28, including the French FRANCE29 and FRANCE 230 registries in which our team was much involved, and clinical indications were extended to bioprosthetic valve failure31. With the increased experience of operators and the development of lower-profile delivery systems, the TAVI procedure was considerably improved. Nowadays, in 90% of cases, TAVI is routinely performed using a simplified transfemoral approach requiring local anaesthesia, a reduced team, fewer resources, and short hospitalisation, an appealing “stent-like” strategy (Figure 12) pioneered and reported by our team for many years32. The celebration in Rouen of the 10th anniversary of the first-in-man was a great event (Figure 13).
Figure 12. Performing simplified (minimalist) transfemoral TAVI as a “stent-like” procedure, Rouen, 2017. From the left, C. Tron, H. Eltchaninoff.

Figure 13. Celebration of the 10th anniversary of TAVI, in the presence of most pioneers, Rouen, 13 May 2012. In the foreground, one of our invited patients having completed eight years of follow-up.
TAVI takes over from surgery in lower-risk patients
Further expansion of TAVI to intermediate-risk patients was in the pipeline after several observational propensity score-matching studies showing comparable survival rates at one year with TAVI and surgical valve replacement33,34. The indication was recently validated by the FDA after the positive results of the randomised PARTNER 2 study with the Edwards SAPIEN XT valve35. This study showed comparable benefit of TAVI on the primary endpoint of death and disabling stroke at two years compared to SAVR, and better results when the femoral approach was used. The superiority of TAVI versus SAVR was even enhanced in the PARTNER 2 S3i propensity score analysis study36 using the latest generation of Edwards valves (SAPIEN 3). With similar endpoints at two years, the recent report of the SURTAVI trial with the Medtronic CoreValve at the 2017 American College of Cardiology sessions also confirmed the non-inferiority of TAVI compared to surgery in this subset of patients37. Of note, TAVI for intermediate-risk patients appears in the updated 2017 AHA/ACC guidelines, with a Class IIb indication. Finally, after the promising results of a first small-size randomised trial published in 2015 (NOTION trial)38, three major randomised trials are currently ongoing in “all-comers”, the upcoming results of which might lead to a broad expansion of TAVI to low-risk patients in the near future. TAVI is on the way to becoming the default strategy for the treatment of aortic stenosis. It can be predicted that within five years the indication for SAVR will be limited to patients who are not optimal candidates for TAVI.
Fifteen years after the first-in-human case, one can say that TAVI, with its explosive potential, represents one of the major medical breakthroughs of the past decade in cardiology. Developing TAVI has been an exciting 25-year odyssey, resulting in a landmark event in medicine, far beyond the initial pioneer’s dream. Let us here quote André Gide, “One doesn’t discover new lands without consenting to lose sight of the shore for a very long time”.
Conflict of interest statement
A. Cribier is a consultant for Edwards Lifesciences.

