
In the early days of stenting, Jacques Puel used to describe the stent as an endoluminal prosthesis. It was Ulrich Sigwart in one of his live courses in 1986 who introduced the term “stented angioplasty”. To convey the concept to an auditorium full of interventional cardiologists, he used to show an image of a gallery in a coalmine scaffolded by balks of wood to explain the concept of scaffolding after balloon angioplasty dilatation. The balloon dilatation creates a “therapeutic dissection” with a fracture of the intima in order to enlarge the lumen1. Unfortunately, in some cases the therapeutic dissection could get out of control and generate an intramural haematoma with the extension of the dissection that could eventually lead to the abrupt occlusion of the vessel. The polymeric scaffold does not have the dilating and stretching properties of the metal, and its function is mainly to maintain the patency of the vessel preventing the collapse of the dissected vessel wall.
Since the initial balloon angioplasty carried out by Andreas Grüntzig in 1977, we have repeatedly seen unexpected issues that initially jeopardise the introduction and adoption of each new technology that we casually call a new enemy. Even the initial and historic balloon angioplasty was seriously challenged by some of our colleagues in the early days after its introduction. In 1982, before the introduction of the long steerable wire, the technique of the Grüntzig balloon was providing a success rate of 70% to 80% with a substantial number of patients suffering vessel closure, leading to myocardial infarction that could only be resolved by urgent coronary artery bypass surgery. Andreas Grüntzig very quickly discovered a new enemy called restenosis in about 30% of his patients2. At that time, in the early 1980s, at a meeting of the European Society of Cardiology in London, the central debate concerned the question “Is percutaneous transluminal coronary angioplasty (PTCA) going to survive or is this therapy going to disappear?”.
Metallic stents were introduced and investigated as a means of treating abrupt coronary occlusions and reducing the growth of intimal hyperplasia. In 1991, we had to admit that the first experience with the stenting technique resulted in acute and late vessel occlusion in nearly one quarter of patients3. Early thrombotic occlusion and late obstructive restenosis due to exuberant neointimal hyperplasia caused by the foreign body were again the new enemies.
As a means to counteract the excessive formation of neointimal hyperplasia, brachytherapy was introduced to subdue the proliferation of smooth muscle cells, which, after phenotypic change, produced a huge number of proteoglycans4. Brachytherapy succeeded in mastering this phenomenon until we realised that late thrombotic events were occurring months after the radiation. The concept of very late stent thrombosis emerged in the year 1999, at that time called “late-late thrombosis”5. Six cases in 100 patients were reported in Circulation; although the paper had been initially rejected by the Editorial Board of the journal, James Willerson, at that time chief editor, reversed his decision when an appeal was sent to the journal6. A new enemy was again defying the field of interventional cardiology. This time it succeeded and radiation came to be considered a “time bomb” for late stent thrombosis5.
By the year 1999, drug-eluting stents (DES) had been introduced on both sides of the Atlantic, in Sao Paulo and Rotterdam. Very quickly we were carried away by the hype of the extraordinary results seen after implantation of DES. In the first 45 patients, restenosis had virtually disappeared7. The enthusiasm was such that together with Cordis and Marie-Claude Morice we planned a randomised trial called RAVEL. In February 2002, the presentation of the results of RAVEL in the Late Breaking Clinical Trials session at the Transcatheter Cardiovascular Therapeutics (TCT) meeting in Washington, DC, USA was a real shock for the interventional community. The numbers spoke for themselves, 0% restenosis in the CYPHER® (Cordis Corporation, Bridgewater, NJ, USA) group and 26% in the BX Velocity (Cordis Corporation) group8. Following CE mark approval, some of us embraced the new technology completely and decided to replace bare metal stents by complete use of DES. The RESEARCH registry is a good example of this phase of extreme enthusiasm. Other colleagues randomised all their patients between the two existing DES (TAXUS™ [Boston Scientific, Natick, MA, USA] and CYPHER®) in the SYRTAX trial9,10. The enthusiasm was general until early 2004 when, within a few weeks, four patients presented with an acute myocardial infarction due to definite late stent thrombosis following the discontinuation of daily aspirin due to non-cardiac procedures. The cause-effect relationship was so evident that the Washington group headed by Ron Waksman and the Thoraxcenter group pooled the data from the four patients in a single manuscript published in the Lancet11. The year after, in 2005, we described what at that time we called LAST (i.e., late stent thrombosis)12. In the meantime, Rotterdam and Bern started to pool their patients in the so-called Bern-Rotterdam registry which described a constant incremental risk of 0.6% stent thrombosis per year up to three years of follow-up13.
In 2006, during the European Society of Cardiology (ESC) congress in Barcelona, the sensationalism around the data showing an increased rate of late stent thrombosis after DES implantation formed the epicentre of the meeting. In the so-called “ESC storm” Edoardo Camenzind and Alain Nordmann presented two meta-analyses suggesting an increased rate of stent thrombosis and non-cardiac death (e.g., cancer) in patients treated with first-generation DES. Most of us will be aware of the consequences of the ESC storm, with multiple publications in the New England Journal of Medicine followed by the meeting of the panel of the FDA in December 2006. At that time, an editorial was published in EuroIntervention about the impact of the FDA panel decision, forecasting long years of research with dual antiplatelet therapy (DAPT) as the consequence of the decision of the panel which recognised the safety of the on-label use but emphasised the unpredictable outcomes of the off-label use of DES14. It took a few years for the interventional community to recover from this affair; nevertheless, after refinements made in the DES technology and improvements in DAPT, DES had regained its safety profile although with no benefit in all-cause mortality compared to BMS15,16.
Then came the era of bioresorbable scaffolds, with the dream that the absence of a foreign body would eliminate the threat of very late thrombosis and would facilitate the easy discontinuation of DAPT. Given that today we can comprehend and manage the phenomenon of acute and subacute thrombosis, it has become evident that once again a new enemy may be around the corner. It is too early to say if there is a real signal in terms of late safety or just a false alarm; however, a recent review of the data available in the literature indicates an incidence of very late scaffold thrombosis of 1.0% (95% CI: 0.6% to 1.5%: 10 studies, 2,331 patients). Although this incidence is low and potentially acceptable, it is clear that it seems to be a step backwards in establishing a bulletproof therapy with predictable and safe long-term follow-up17. With the two-year results of the ABSORB II and ABSORB Japan trials published in this issue of EuroIntervention, the hazard ratio for very late scaffold thrombosis is in the range of 3.4 versus the metallic DES (Figure 1)18,19. At the same time, we have also to acknowledge the excellent performance of the newer generation of DES (i.e., everolimus-eluting stents) which were used as a control group in these trials.
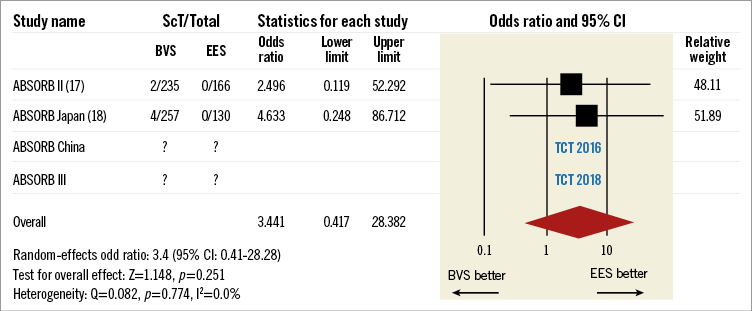
Figure 1. Very late scaffold thrombosis.
This year at the TCT meeting, brand new information in the field of coronary revascularisation will be presented. The EXCEL and NOBLE trials will tell us if we have made progress in the percutaneous treatment of main stem disease. Also, new information on the bioresorbable scaffold will become available with the presentation of the three-year results of the ABSORB II trial and two-year results of the ABSORB China study. We will have to digest all of this information and go forward to improve the outcomes of our patients further without ignoring the extraordinary progress of pharmacotherapy in the field of coronary atherosclerosis treatment.
Conflict of interest statement
P.W. Serruys is a member of the International Advisory Board of Abbott Vascular. C. Collet has no conflicts of interest to declare.

