In its 37-year existence, the subspecialty of interventional cardiology has achieved stepwise improvements in patient outcomes through evolution of its core technology, from balloon angioplasty to bare metal stents (BMS) and now to drug-eluting stents (DES). Moreover, the current generation of thin-strut fluoropolymer-based everolimus-eluting stents (EES), slow-release zotarolimus-eluting stents and drug elution from bioresorbable polymer-based metallic DES have substantially improved safety and efficacy compared to both BMS and first-generation DES1. Nonetheless, real-world stent thrombosis (ST) rates are still unacceptable, and restenosis in complex patients and lesions within the first year is still excessive2. Less well appreciated is the fact that very late (>1 year) rates of target lesion failure (TLF; the composite of death, target vessel myocardial infarction, or ischaemia-driven target lesion revascularisation) occur in 1-2% of DES-treated patients per year, at least through five years of follow-up, with no plateau evident. In contrast, the clinical results of coronary artery bypass graft (CABG) surgery are more durable. Thus, in the SYNTAX trial, the absolute difference in major adverse cardiac and cerebrovascular events favouring CABG over paclitaxel-eluting stents (PES) was 5.4% at one year (12.4% vs. 17.8%, respectively) and 10.4% at five years (37.3% vs. 26.9%, respectively)3. Furthermore, current-generation DES have not improved upon these late results. For example, in the SPIRIT III and SPIRIT IV randomised trials, whereas EES had substantially lower TLF rates than PES at one year, 1.5-2.0% of patients/year developed TLF between years two and five, independent of stent type4,5. For these reasons even the best metallic DES are unlikely to match the long-term outcomes of CABG in very complex patients. Moreover, comparable annual rates of very late TLF events are seen for at least 15 years after BMS6, suggesting a commonality of this phenomenon to metallic stents in general.
The aetiology of very late TLF events (both ST and restenosis) after metallic stents is likely to be multifactorial. Lack of complete endothelialisation contributes to ongoing ST risk, a mechanism which has been reduced but not eliminated with contemporary DES7. Polymer hypersensitivity and foreign body reactions can result in chronic inflammation, positive remodelling, acquired strut malapposition and late events7. Excessive uptake of circulating lipid may transform in-stent neointimal tissue from a stable to an unstable phenotype prone to expansion and thrombosis (neoatherosclerosis), an increasingly recognised cause of very late stent failure8. Strut fracture (which has not been eliminated with current thin-strut stents) can lead to very late restenosis and occasionally aneurysm formation or ST9. Contributing to these mechanisms may be loss of normal vasomotor responses at and distal to the stent site; persistent stimulation of smooth muscle cells from adherent fibrin and/or loss of normal vessel curvature; and abnormal shear stress from protruding struts and/or loss of cyclic strain relief (compliance mismatch).
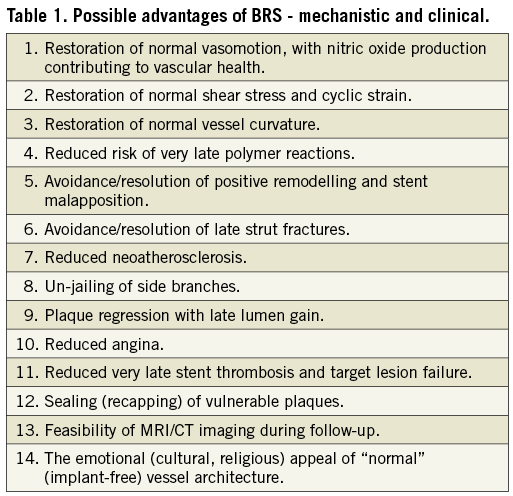
Enter the class of coronary bioresorbable scaffolds (BRS), devices designed to accomplish the same goals as metallic DES within the first year after implantation (seal dissections, prevent acute recoil and constrictive remodelling, and suppress neointimal hyperplasia), thereafter disappearing entirely, restoring the underlying vasculature to its native pristine state (at least to the extent possible given the structural changes of chronic atherosclerosis). Although sharing a similar configuration with its metallic predecessors, the temporary nature of this innovative technology is reflected in the fact that it is no longer even termed a stent, but rather a scaffold. Current BRS are being formulated from fully bioresorbable polymers (most commonly poly-L-lactic acid [PLLA], although BRS comprised of poly-DL-lactic acid [PDLLA], tyrosine-derived polycarbonates, and salicylate-based materials are also being tested) and metals (magnesium and iron alloys), each of which varies in terms of its mechanical properties, propensity for inflammation, and resorption/healing characteristics. BRS are inherently more prone to inflammation than metallic DES using non-erodible polymers or lower loads of bioresorbable polymers, and tend to have thicker struts to preserve mechanical strength; as such it is doubtful that they will improve upon the one-year rates of event-free survival achieved by contemporary metallic DES. Rather, it is hypothesised that the chemical, biological and mechanical mechanisms inciting very late events will fade along with the device, and as such restenosis and ST beyond one year will become increasingly less frequent. Potential advantages of BRS appear in Table 1.
While several BRS have already achieved CE mark and are in clinical use in most global geographies (the USA being a notable exception), the majority of what we know about BRS in humans has emanated from a series of meticulous imaging studies performed by Patrick Serruys, John Ormiston and their colleagues in two versions of the Absorb PLLA-based everolimus-eluting scaffold (Abbott Vascular, Santa Clara, CA, USA) in 30 patients (v1.0) and 101 patients (v1.1), the latter representing the currently manufactured device. The vascular responses to these devices have been assessed using quantitative and qualitative angiography (including vasomotion assessment), greyscale and radiofrequency ultrasound imaging, optical coherence tomography, and computerised tomographic angiography at pre-specified intervals through five-year follow-up. The current issue of EuroIntervention contains the most comprehensive report to date of the three-year results with the Absorb v1.1 device10, which has been implanted in >30,000 patients
to date. The findings demonstrate: 1) progressive bioresorption through three years as the polymer is replaced by cellular and organic material (although imaging studies are unable to delineate exactly when this process is complete in situ, whether before, at, or after three years); 2) increasing scaffold area after one year signifying strut discontinuity; 3) increasing plaque burden through two years with a corresponding adaptive increase in vessel dimensions, followed by plaque regression and reduction in vessel dimensions between years two and three (the net effect preserving or even allowing the lumen area to enlarge further during this dynamic period) – these changes do not occur with metallic stents; 4) a low rate of angiographic late loss at one year (similar to EES), without incremental late loss through three years (in contrast to increasing late loss over time with EES)11; and 5) restoration of vasomotion beginning at one year and increasing in frequency and magnitude over the three-year follow-up period. The number of patients studied is too small to draw inferences from the observed Absorb clinical event rates, although they are roughly in accordance with what would have been expected from EES in comparable patients.
In the mid 1980s Andre Agassi burst upon the tennis scene and captivated a new generation with a unique style epitomised by the catchphrase “Image is Everything”. However, it was not until years later when Agassi started to win grand slam tournaments that his true genius as a tennis player was appreciated, and his legacy cemented. Similarly, the dramatic images and unique vascular responses after BRS implantation, as brilliantly elucidated by the imaging studies of Serruys and colleagues, are fascinating the subspecialty of interventional cardiology – we have never seen anything like this before, and the clinical potential for this innovative technology is alluring. However, our excitement must be tempered by the recognition that, compared to contemporary metallic DES, current BRS may have greater crossing profiles and reduced deliverability; thicker struts with the potential for greater side branch compromise and (theoretically) delayed re-endothelialisation; a more limited expansion range (with increased susceptibility to fracture); less secure retention on the delivery balloon; and somewhat greater recoil requiring more aggressive lesion preparation. Although these properties will no doubt be improved with future device iterations, fulfilment of the promise of BRS awaits the results from large-scale randomised trials demonstrating clear-cut clinical benefits. Toward this end, the ABSORB III and IV trials are randomising 5,000 patients to Absorb vs. EES, powered to demonstrate reduced TLF with Absorb between one and five years of follow-up. Other objectives are to establish non-inferiority between the two devices at one year, and superiority of Absorb with respect to angina relief. If these goals are met, Absorb (and the conceptual framework of BRS) will become established as the fourth revolution in interventional cardiology, and the therapeutic landscape for patients with atherosclerotic coronary artery disease will be radically transformed.
Conflict of interest statement
The author is a consultant to Boston Scientific, and to REVA Medical Inc.

