
Over the last 15 years, the science of the bioresorbable scaffold (BRS) has evolved from a seemingly unattainable dream to an exciting reality. Although there is a variety of BRS in various stages of development, by far the most successful of these with the largest amount of robust scientific and clinical data is the Absorb™ Bioresorbable Vascular Scaffold (A-BVS) (Abbott Vascular, Santa Clara, CA, USA). A number of prospective registries and pivotal trials have affirmed that the short-term safety and efficacy of this device is equivalent to the “best in class” metallic drug-eluting stent (mDES)1,2. However, its longer-term “real benefits” in relation to the lack of a permanent implant will need to be proven over time.
Despite the revolutionary science, the uptake of BRS by the interventional community has been slow. While some continue to enthusiastically explore the “uncharted rough seas” by using this device in very complex “real-world” patients3, many are cautiously sailing the calmer waters of simpler lesions, waiting for someone to draw out the guiding maps. Still others are on the seashore wondering and waiting for the right “tide” to catch them. The recent report of increased scaffold thrombosis rates in complex “real-world” patients from the GHOST-EU registry4 has been a “storm in the sea”, discouraging many of the “half-hearted”.
Thus, the article by De Ribamar Costa et al5 in this issue of EuroIntervention is appropriately timed to provide a “focus” on some of the relevant procedural issues concerning BRS in order to achieve the best outcomes. The authors looked at the deployment technique of the A-BVS in 768 patients enrolled in the ABSORB EXTEND study6 and compared the one-year outcomes of the 526 patients (68%) who underwent post-implantation balloon dilatation to the 242 patients (32%) who did not undergo post-implantation balloon dilatation. At one year, there was no significant difference in the rates of ischaemia-driven target lesion revascularisation (TLR) (1.6% vs. 1.2%) or scaffold thrombosis (1.6% vs. 1.2%) between the two groups. However, there was a significantly higher rate of procedural, protocol-defined, enzymatic non-Q-wave myocardial infarction (MI) in the post-dilatation group (2.7% vs. 0%, p=0.007), which had no impact on death, Q-wave MI or TLR at one year. Therefore, the authors concluded that performing post-dilatation does not negatively influence long-term clinical outcomes and should be performed whenever necessary to optimise acute results. To be fair, some readers may disagree with this conclusion, noting that if post-dilatation did not provide any outcome advantages in the first place, then why do it at all?
The answer is that the ABSORB EXTEND cohort represented a relatively “non-complex” lesion population (mean lesion length 12.3 mm, 93% single lesions, and only 9% overlapping stents in relatively straight, non-severely calcified, non-severely angulated and non-ostial lesions in stable patients). When we extend the use of BRS to complex “real-world” patients with long lesions, diffuse disease, calcified lesions, bifurcation disease, chronic total occlusion, arterial reconstruction with “full plastic jackets”, and those with acute coronary syndromes; high-pressure post-dilatation can really make a difference.
It was this technique of “high-pressure post-dilatation”, as evaluated by the IVUS study of Jonathan Tobis and Antonio Colombo in 19957, which achieved optimal expansion and the largest possible acute gain, and led to a decrease in acute stent thrombosis in the short term and restenosis at follow-up, thus establishing the first generation of metallic stents as the second revolution in interventional cardiology.
The A-BVS in its current form is a 156 μm thick strut device, similar to the first-generation metallic stents, and almost twice as thick as the present generation of mDES. Thus, it takes a longer time to be covered by neointima, and may be more thrombogenic and less forgiving in its acute phase of implantation when compared to the thin-strut third-generation mDES. Underexpansion has been another important reason for acute stent thrombosis in many studies8, and the same holds true for BRS. Hence, meticulous attention to the optimal implantation technique in order to achieve complete expansion and apposition is vital in complex “real-world” cases. For us, this involves good lesion preparation by adequate predilatation with a near optimal size non-compliant balloon to high pressures, the adjunctive use of cutting balloons, scoring balloons or rotational atherectomy for calcified and fibrotic lesions, accurate sizing of BRS to the vessel diameter (when in doubt a 0.5 mm larger BRS) and high pressure post-dilatation with a quarter size larger non-compliant balloon to ≥18-20 atm. Liberal use of intravascular imaging, especially during the learning curve, helps to refine and redefine the vessel sizing and optimal deployment technique for the operator, but, once experience is gained, its routine use to guide adequacy of result in all cases is arguable. Utilising this meticulous technique of implantation and high-pressure post-dilatation, Mattesini et al9 demonstrated by OCT that stent performance indexes of minimal area, residual area stenosis, incomplete strut apposition, tissue prolapse, eccentricity, and symmetry for BRS could be similar to a second-generation mDES. Costopoulos et al10 published their experience of 92 patients treated with 137 A-BVS and compared them to a propensity score-matched population of 92 patients treated with cobalt-chromium everolimus-eluting stents in a very complex “real-world” population (84% B2 and C lesion, 21% severely calcified, 45% bifurcations). Post-dilatation was performed in 99% of patients to maximum inflation pressures of 21 atm. MACE at six months was 3.3% in the A-BVS group versus 7.6% in the EES group. Most importantly, there was no stent thrombosis in either group up to six months. Others, including ourselves, have had a similar experience of extremely low stent thrombosis and MACE rates similar to mDES in a very complex lesion population when using the optimal deployment technique and high-pressure post-dilatation11. In the GHOST-EU registry performed in 2011-2013, scaffold thrombosis was 2.1% at six months. Twenty-two percent of the scaffold thrombosis occurred within 24 hours and 70% occurred within 30 days, suggesting a non-optimal deployment technique as the probable cause of early stent thrombosis. Only 46% of patients had post-dilatation performed and only 20% underwent intravascular imaging.
We have regularly demonstrated by OCT that, despite adequate and aggressive predilatation of the lesion and subsequent deployment of a BRS to achieve a good angiographic result (Figure 1), a high-pressure post-dilatation with a non-compliant balloon 0.25 mm larger (while achieving the same final size as the A-BVS implant balloon as per pressure compliance data chart) still corrects underexpansion, apposes struts, achieves better luminal enlargement and even embeds the thick struts into the vessel wall (Figure 2). All of this helps to decrease stent thrombosis and improve outcomes. While some might argue that one should image all BRS implantations and post-dilate where inadequacies are noticed, we would advise “high-pressure post-dilatation” in all cases and image a few extremely complex anatomies (such as full plastic jacket, bifurcations with two-scaffold strategies, heavily calcified lesions or diagnostic dilemmas). This approach is more practical for the interventional community across the world to be able to use BRS safely and benefit their patients.
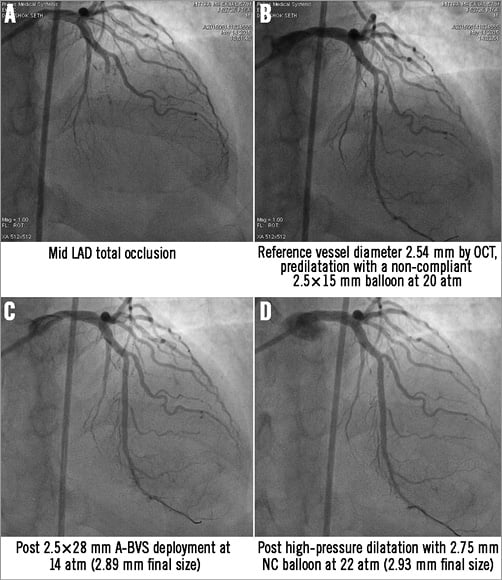
Figure 1. Angiographic image of mid LAD lesion treated with a 2.5×28 mm A-BVS showing similar angiographic appearance before and after high pressure post-dilatation.
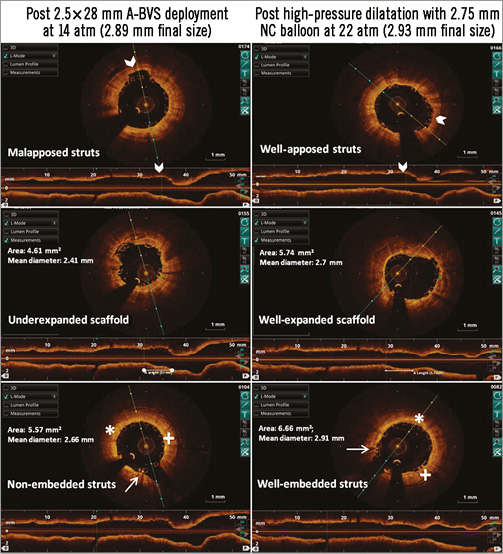
Figure 2. OCT images of the same mid LAD lesion showing inadequate deployment of A-BVS which is optimised by high-pressure non-compliant balloon post-dilatation despite achieving similar final balloon diameters.
Finally, we have to realise that A-BVS in its present form is not a stent but a “new device”. It has a unique structure, different performance characteristics, and specific expansion and deployment parameters. It therefore requires a fresh set of tips and tricks for complex lesion anatomies to which we would strongly recommend adding “high-pressure post-dilatation”. In a way, for many of us, this feels like déjà vu of 20 years ago when we first learnt how to implant a device safely into human coronary arteries by using high-pressure post-dilatation.
Conflict of interest statement
A. Seth was the lead investigator for India in the ABSORB EXTEND study and is a member of the Global Strategic Advisory Board for BVS, Abbott Vascular. The other authors have no conflicts of interest to declare.

