After the first positive results for the treatment of coronary lesions using bioresorbable scaffolds (BRS), the Bioresorbable Scaffolds versus Metallic Stents in Routine PCI (AIDA) trial and the three-year clinical outcomes of the ABSORB III trial have demonstrated an increased risk of scaffold thrombosis using the Absorb™ BRS (Abbott Vascular, Santa Clara, CA, USA) compared with the conventional everolimus drug-eluting stent in contemporary cohorts of patients1,2. When implanting current Absorb BRS important considerations need to be kept in mind. The device is, by structure design and performance, more thrombogenic than current second-generation drug-eluting stents. The only way to make the performance of the Absorb BRS non-inferior to current DES is to optimise the immediate stenting procedure (IVUS, predilatation, post-dilatation, IVUS, etc.) and to utilise the most aggressive drugs to inhibit platelet aggregation. The causes of the higher rate of thrombosis with the Absorb were only partially understood and some concerns have been raised about the optimal preparation of the lesion and insufficient post-dilatation, as the residual diameter stenosis was at least 30% in patients with BRS thrombosis1. Longer rather than shorter dual antiplatelet therapy may be required, considering that 79% of patients with BRS thrombosis did not receive dual antiplatelet therapy at the time of the event1. Considering the lack of advantage with respect to clinical safety, difficulties in scaffold deliverability, longer procedural times and the devices needed to ensure an optimal final BRS implantation, little justification exists to prefer these devices over modern and safe drug-eluting metallic stents. The new guidelines on coronary revascularisation discourage the use of BRS outside of clinical studies with a class III level of evidence C recommendation3. However, the guidelines statement is supported only by clinical data on the Absorb and does not take into account data about newer generations of BRS. New-generation BRS have promising futures that could contribute to cutting the rate of adverse clinical events observed in previous trials. New devices with a smaller footprint, less thrombogenicity (e.g., magnesium), faster reabsorption and advanced mechanical properties had to overcome the limitations of the Absorb scaffold (Figure 1).
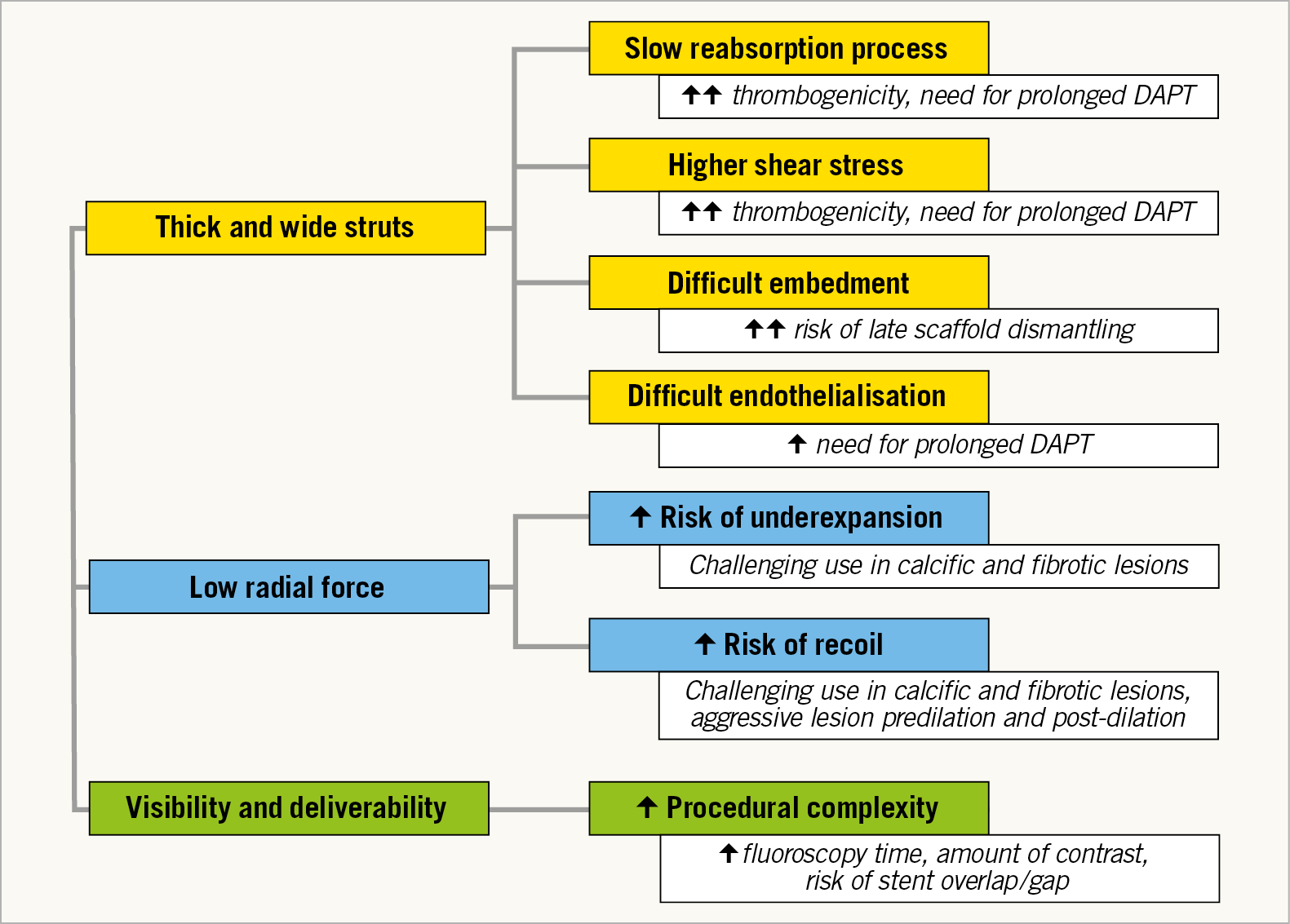
Figure 1. Challenges of the first-generation bioresorbable scaffold. DAPT: dual antiplatelet therapy
As pointed out by Katagiri et al, new studies with the next-generation BRS show promising results and, even if no long-term follow-up is available, we should be optimistic about the future of the new-generation BRS, removing the negative concept of “class effect” applied to BRS4. Moreover, a meticulous lesion preparation has also been demonstrated to reduce the rate of device-related clinical adverse events at long-term follow-up5.
Gheorghe et al report the long-term follow-up of 33 chronic total occlusions (CTO) treated with Absorb BRS using a dedicated implantation technique6. At three years, the rate of target lesion failure was 3%; an increase of the scaffold diameters from 12 to 36 months was observed, thus indicating a possible positive vessel remodelling with vasomotion documented in 70% of the lesions.
Insights from the BVS STEMI STRATEGY-IT study by Hioki et al confirmed the good results obtained with the predilation, sizing and post-dilation (PSP) implantation technique plus thrombectomy in BRS implants during acute coronary syndromes with ST elevation7.
Even if no clear advantage was observed at one-year follow-up, the dedicated implantation technique was significantly associated with better post-procedural minimum lumen diameter and maximum footprint. This finding reinforces the concept that good lesion preparation, meticulous implantation supported by intracoronary imaging and an accurate post-dilation reduce the rate of BRS-related clinical events5. The BRS technology may be ready for a “step-by-step” comeback, as suggested by Katagiri et al. We believe that the recommendations of the new guidelines do not help the development of new BRS technologies, put strong limitations on the use of BRS platforms (even with the Conformité Européenne [CE] mark), thus discouraging the collection of clinical experiences with newer platforms. The interventionalist should be aware of the possible risks related to the use of this technology but not discouraged from using “new-generation BRS” that have been shown to give new hope for the future development of this technology. We cannot dismiss the need for new BRS technology that can be utilised with the same implantation steps as current metallic stents with non-inferior immediate and long-term event rates. In addition, we need to clarify the role of resorption and thrombosis by defining the optimal duration of dual antiplatelet therapy. Some of these attributes are present in new-generation BRS8, limiting current concerns to the Absorb device.
Conflict of interest statement
The authors have no conflicts of interest to declare.

