Introduction
The concept supporting bioresorbable scaffolds (BRS) was to provide the benefits of stenting without leaving a permanent implant behind. However, target lesion failure and device thrombosis were important concerns with first-generation BRS, leading to low rates of adoption in clinical practice. New iterations, incorporating improvements such as a smaller strut thickness have been proposed and evaluated in early investigations. However, it is still unclear whether these modifications are sufficient to achieve outcomes comparable to those of metallic drug-eluting stents (DES) while preserving the benefits of resorption.
Pros
Gregg W. Stone, MD; Solomon W. Biensock, MD
Fully bioresorbable scaffolds were designed to provide the mechanical support and drug delivery functions of metallic drug-eluting stents in the first year and then completely resorb within 2 to 4 years, removing the nidus for very late adverse events including stent thrombosis and restenosis. BRS also address the practical limitations of metallic stents by “unjailing” covered side branches, “unjacketing” long treated segments (thereby preserving late coronary artery bypass grafting options), “unlayering” treated in-stent restenosis, eliminating artefacts with non-invasive imaging (e.g., computed tomographic angiography), and addressing the individual patient’s cultural, religious, or personal preferences to not have a permanent implant, a more frequent concern than is widely realised.
Investigational studies and case reports have demonstrated that these objectives have been met. BRS designed from polymers or absorbable metals have been shown to completely resorb within 1-4 years, restoring vascular compliance and remodelling capabilities. It is quite remarkable to view a heavily diseased coronary artery that 5 years after treatment with a BRS is widely patent and minimally diseased, with no metallic stent or other support structures visible by intravascular imaging (Figure 1)! Interventional cardiologists are unanimous in preferring this outcome if it can be achieved as safely and effectively as with metallic DES.
Unfortunately, this promise collided with reality. The ABSORB II, ABSORB III, ABSORB CHINA, and ABSORB JAPAN multicentre, prospective, randomised trials compared the everolimus-eluting poly-L-lactic acid (PLLA)-based Absorb BRS (Abbott Vascular) with a contemporary cobalt-chromium everolimus-eluting stent (CoCr-EES). Adverse ischaemic events, namely target lesion failure (TLF) and device thrombosis, were more common with this first-generation BRS; however, the period of excess risk ended at 3 years after the complete bioresorption of the scaffold. Thereafter, event rates were similar or lower with BRS1. Data have emerged that the use of optimisation techniques, such as “PSP” (preparing the lesion aggressively, sizing the scaffold correctly, and post-dilating at high pressure in all cases), when implanting the first-generation thick-strut Absorb BRS can improve results. The large-scale ABSORB IV trial included mandatory pre- and post-dilation, oversizing by ≤0.5 mm, and strict avoidance of small vessels (<2.25 mm reference diameter by quantitative measurement). The trial demonstrated the non-inferiority of BRS compared with CoCr-EES for TLF at one year2. Nonetheless, 30-day and 1-year rates of device thrombosis and target lesion revascularisation trended higher with Absorb than CoCr-EES. The 5-year results from this trial will be reported this spring. Of note, even in the ABSORB IV trial, intravascular imaging guidance (e.g., with optical coherence tomography [OCT]) for BRS implantation was used infrequently, a technique that may be especially useful to ensure that maximal expansion is achieved and acute malapposition does not occur, the latter a potentially important driver of late-acquired scaffold dismantling and thrombosis3.
Aside from suboptimal technique, there is no doubt that the design characteristics of first-generation BRS impacted its acute and late outcomes. In particular, the strut thickness of the Absorb (>150 μm), almost twice that of metallic stents, resulted in greater luminal protrusion and turbulent flow, delayed endothelialisation, and increased neointimal hyperplasia during follow-up compared with thinner-strut metallic DES12. The Absorb also had a limited expansion range (beyond which fracture could occur) and was prone to acute and occasionally late recoil.
The introduction of thinner-strut resorbable scaffolds with superior mechanical performance offers the potential for BRS technology (especially if implanted with intravascular imaging guidance) to achieve metallic DES-like clinical outcomes within the first several years after implantation, with equal or superior late results, representing a preferred long-term approach to coronary artery disease (CAD). For example, the Firesorb (MicroPort) BRS is a thinner-strut (100-125 μm) PLLA-based sirolimus-eluting BRS designed to decrease luminal protrusion and improve blood flow dynamics. In the FUTURE-II randomised trial (n=433), the Firesorb BRS demonstrated nearly identical 1-year angiographic in-segment late loss and tissue strut coverage by OCT as the CoCr-EES, with 0.9% and 1.9% target lesion failure rates, respectively, with no scaffold thromboses4. Abbott Vascular has developed a future-generation PLLA-based scaffold (Esprit) that has <100 μm strut thickness and is currently being investigated in patients with peripheral vascular disease (ClinicalTrials.gov: NCT04227899). A favourable experience from this trial may lead to a re-emergence of this technology in patients with coronary atherosclerosis. Finally, a preclinical evaluation of an iron-based resorbable scaffold (IBS) with a strut thickness of 70 μm has shown promising results. This novel device has been shown to have comparable operating characteristics and similar midterm (1-year) efficacy and safety as the CoCr-EES, with an acceptable corrosion profile in porcine coronary arteries5.
Given the history with the first-generation BRS, large-scale randomised trials are required before the clinical community will adopt the next generation of devices. However, we predict that should such trials demonstrate even comparable early and late outcomes (let alone superiority), a mass migration away from permanent metallic cages to this more natural and holistic solution would occur. The development of thin-strut fully bioresorbable scaffolds implanted in appropriately selected lesions in a standardised fashion utilising PSP techniques, and, ideally, with OCT imaging guidance, has set the stage for the resurgence of coronary BRS. Industry and physician-scientists just need the courage to take the next leap forward!
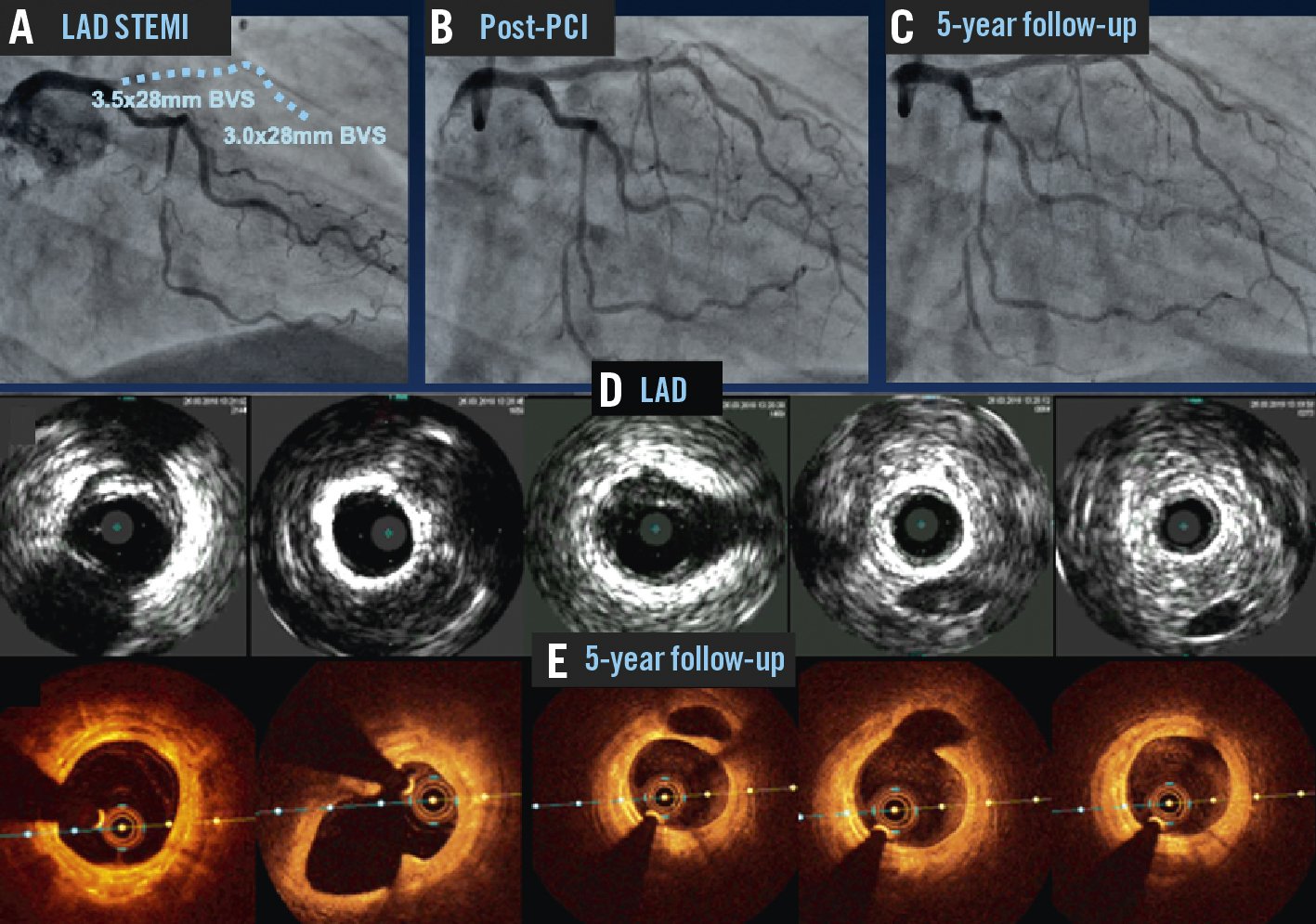
Figure 1. The long-term promise of bioresorbable scaffolds. Reproduced with permission from11. A patient presented with a STEMI. Angiography demonstrated occlusion of the ostial LAD (A). PCI restored patency, and two Absorb BVS were implanted in the proximal and mid-LAD (B). Five years later repeat coronary angiography demonstrated wide patency of the scaffolds (C). Intravascular imaging with ultrasound (D) and optical coherence tomography (E) at 5 years showed minimal neointimal hyperplasia and native atherosclerosis with no residual polymeric scaffold structures. The distal left circumflex artery and distal right coronary artery had also been treated with a drug-eluting balloon and a BVS, respectively, with optimal angiographic results at five-year follow-up. BVS: bioresorbable vascular scaffold; LAD: left descending artery; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction
Conflict of interest statement
G. Stone has received speaker honoraria from Medtronic, Pulnovo, Infraredx, Abiomed, Abbott, and Amgen; has served as a consultant to Daiichi Sankyo, Ablative Solutions, CorFlow, Apollo Therapeutics, Cardiomech, Gore, Robocath, Miracor, Abiomed, Valfix, TherOx, HeartFlow, Vectorious, Neovasc, Ancora, Elucid Bio, Occlutech, Impulse Dynamics, Adona Medical, Millennia Biopharma, and Oxitope; and has equity/options from Ancora, Cagent, Applied Therapeutics, the Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, and Xenter. Dr Stone’s daughter is an employee at IQVIA. S. Biensock has no conflicts of interest to declare.
Cons
Franz-Josef Neumann, MD, PhD, FESC
In percutaneous coronary intervention (PCI), the concept of securing the angioplasty result without leaving behind a permanent foreign body is highly attractive to patients and physicians alike and intuitively promises a number of advantages. However, safety issues with the most extensively studied bioresorbable scaffold (BRS) to date, the Absorb (Abbott Vascular), have challenged the technology. Since then, intense research activity has continued with advances in both polymer-based and metal-based BRS technology6. The question arises as to whether modern BRS are now ready to make the leap from investigational devices to devices with an established niche in clinical practice.
The answer to this question depends on the criteria applied. Any new device for PCI must be assessed against the therapeutic goals of PCI. These are improved survival (a goal that may not be reached in many cases), prevention of myocardial infarction and sustained symptomatic improvement that avoids reintervention. These goals should be achieved with minimal off-target complications, such as bleeding due to extended antiplatelet therapy. To enter into routine clinical practice, any new device must serve these goals better than the standard of care. Alternatively, if a new device simplifies the procedure or consumes fewer resources, non-inferior efficacy may be acceptable. However, BRS are procedurally more demanding and resource-intensive. Therefore, we need at least reasonable evidence, if not proof, that BRS improve the outcome of PCI.
The Absorb did not achieve this goal. The initial registries generated tremendous enthusiasm, and preliminary 1-year results from a randomised trial were hailed for suggesting a symptomatic benefit despite a signal of increased risk of scaffold thrombosis. When the 3-year results became available, the incidences of device thrombosis, target vessel myocardial infarction and reintervention after Absorb implantation were significantly higher than after DES implantation7. These findings have been confirmed by subsequent larger randomised trials3 including those with optimised implantation techniques8. This risk of the Absorb was confined to the biosorption phase1. Conversely, the foreign body-free segment thereafter did not confer a lower risk than the stented segment1. When the Absorb was finally withdrawn, a substantial number of patients had already been treated with this BRS, causing myocardial infarctions and reinterventions that would otherwise have been avoided.
Of course, the results obtained with the Absorb cannot be transferred to current designs. However, the lessons learned from the ABSORB trials apply. They tell us that registries can be misleading and that adequately powered randomised trials are needed that cover at least the biosorption phase. With most current scaffold designs, however, investigations are still at the registry level. Three randomised trials4910 with different polymeric BRS included fewer than 500 patients each with 1 or 3 years of follow-up. The respective BRS did not outperform the comparator DES4910. Even the trial with 3-year follow-up cannot exclude a signal of harm due to limited power10. In two of these trials49, in-device acute gain was significantly lower and in-device late loss was significantly,9 or numerically4, higher with BRS than with DES. For the magnesium scaffold, promising data are available from several registries. Nevertheless, a single, small, randomised trial showed no benefit in terms of 3-year survival without myocardial infarction but a significantly increased need for reintervention.
If we accept the criteria outlined above, the evidence currently available is insufficient to justify the implantation of BRS outside of clinical trials. It is a common paradigm in the history of medicine that enthusiasm for an intellectually appealing concept is fuelled by registries and small randomised studies but then dashed by larger, appropriately designed trials. Given the efficacy and safety of current DES, even with reduced antiplatelet therapy, there is no urgent need to run this risk again.
Conflict of interest statement
F.-J. Neumann reports consultancy fees from Novartis and speaker honoraria from Amgen, Boston Scientific, and Daiichi Sankyo; speaker honoraria paid to his institution from Bayer Healthcare, Biotronik, Boehringer Ingelheim, Edwards Lifesciences, Ferrer, and Pfizer; consultancy fees paid to his institution from Boehringer Ingelheim; and research grants from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.

