Why bioresorbable stents?
More than 20 years ago, interventional cardiology was launched by Andreas Grüntzig, introducing a revolutionary innovation, i.e., percutaneous balloon angioplasty1. The 316L stainless steel stent was initially introduced to tackle acute closure secondary to dissections following balloon angioplasty. In fact, the stent not only resolved the acute complications, but also appeared to be the appropriate response to the midterm complication, i.e., restenosis2,3. Indeed, the principal mechanism of restenosis after balloon angioplasty appeared to be related to chronic constrictive remodelling as a negative component of the healing process4-6. Nevertheless, the permanent (316L stainless steel) stent was the origin of a new disease, in-stent restenosis, corresponding to a foreign body reaction. Drug eluting stents were conceived to skip this particular healing process via cell cycle-inhibiting drugs in order to prevent in-stent restenosis. However, these drugs were cytotoxic, not only against smooth muscle cells, but also endothelial cells leading to late stent thrombosis and/or restenosis7,8. So, by inhibiting this inescapable healing process, we have delayed the occurrence of complications. The approach of Arterial Remodelling Technologies (A.R.T.) is to consider that the error resides in the discrepancy between the healing duration (i.e., a few months) and the immortal half life of the metallic stent, the source of new complications when it is no longer necessary: the bioresorbable stent acts as a transitory cast, scaffolding the artery wall during the healing process. Moreover, the healing process will induce endothelial coverage, a mandatory step for the mid- and long-term follow-up that will simplify one of the issues of drug eluting stents, i.e., expensive and prolonged dual antiplatelet therapy reducing the quality of life. The degradation of the bioresorbable stent will result in stent fragmentation, stopping the perpetual mechanico-genic stimulus produced by the stent’s overstretching of the arterial wall after the healing process. This can then restore the remodelling capacities of the artery wall itself, thus preserving its future, avoiding a growing accumulation of metal in the arteries9,10. Last but not least, the bioresorbable stent will not impair vasomotion after healing, namely at its extremities.
A.R.T.: where do we come from?
A.R.T. refers to the original findings of our group, i.e., the major impact of arterial remodelling after angioplasty during the healing process. Originally, this concept was discovered from findings coming out after the first experimental restenotic model which was determined at the Cleveland Clinic Foundation in the 90’s; questioning the admitted role of neointimal hyperplasia and demonstrating the importance of chronic constrictive remodelling on restenosis after balloon angioplasty4. As a consequence, the idea of a transitory stent appeared as the optimal therapeutic solution, a solution that was developed in collaboration with F. Cornhill, head of the Bioengineering Department of the Cleveland Clinic Foundation. At the time, J. Anderson, a well known expert of biodegradable polymers at Case Western Reserve University, when approached about a potential collaboration, suggested to Antoine Lafont, who was about to go back to Paris and Descartes University, to meet with M. Vert, at the French Centre National de la Recherche Scientifique (CNRS) in Paris, concerning Mr. Vert’s well known expertise on the utilisation of biopolymers in the medical field. This represents the rationale of the collaboration between these three institutions – the Cleveland Clinic Foundation, Paris Descartes University, and the CNRS – originally at the scientific level, on the very concept of a bioresorbable polymer itself. Antoine Lafont received his PhD under the authority of M. Vert, his thesis concerned the use of bioresorbable polymers for vascular stents11. A patent was then obtained by the three institutions, and a start-up company A.R.T. was founded by F. Cornhill, Antoine Lafont and M. Vert in 2002, with funding obtained from French venture capitalists.
Bioresorbable stents: what are the issues?
Bioresorbable stents face three issues that present multiple interactions: the biomechanics, the bioresorption, and the biocompatibility. The A.R.T. strategy has been to comply with these three different dynamics – their interactions – taking advantage of the specific properties of the polylactic acid. This approach is time-consuming, but represents the necessary step-by-step progression in order to go from the metal to the bioresorbable stent. Indeed, a bioresorbable stent must have biomechanical properties that will integrate the timing of the resorption, the alteration of the polylactic acid (PLA) i.e., plasticity, hydration, molecular weight12,13. It will also need to have a bioresorption process following the first phase (mechanical scaffolding), the second phase (stent dismantling), and the third phase (stent disappearance). The choice of PLA is based on its excellent tolerance in vivo in several medical applications14-17. Eventually, the stent will have to remain biocompatible throughout the three phases, despite PLA chemical alterations during the resorption, and the healing process of the artery wall. Thus, the complexity of this stent is to integrate simultaneously these three indispensable aspects; below we will develop each of these three individually, describing them in detail.
The A.R.T. concept
Our concept is to simultaneously balance biocompatibility, biomechanics, and bioresorption in a bioresorbable PLA stent without altering healing by drug elution from the stent platform. We provide a transient effective scaffolding for five to seven months, followed by a complete monomer resorption within 18 months. The stent applies conventional delivery, is balloon expandable, and 6 Fr compatible. The extensive investigation has taught us that quality preservation of the PLA is essential and critical to achieve the optimal balance. The excellence of this superior biocompatible oligo-element initiated PLA is preserved throughout the whole processing, providing very limited transformation/degradation measured by molecular weight (Mw) loss of less than 15% from granules to sterilised product.
Biomechanics
The biomechanics of polymer stents has been extensively evaluated12,13. Classically, recoil has been an issue of bioresorbable stents which has recently been seen with a bioresorbable stent showing a slight acute recoil in human coronary arteries18. Moreover, optical coherence tomography revealed ellipsoid forms of the stents in contrast with metallic stents. The magnesium stent suffered from severe recoil resulting in restenosis in humans19. PLA can be compared to a stiff sponge when dry, which becomes soft when it is hydrated. Initially, we encountered similar patterns that could be attributed to both an alteration of the mechanical properties of the stent in vivo related to excessive hydration, loss of radial force leading sometimes to stent collapse. Interestingly, this did not lead to acute closure, and the collapsed stent was embedded under an endothelialised cap by the healing process. The other reason for stent recoil was the occurrence of stent strut fractures either during the stent crimping process, or secondary to deployment. Moreover, it appears that polymers quickly lose their molecular weight before its use, throughout the successive steps from the granular state to the stent sterilisation. This loss of molecular weight indicates an alteration of PLA quality, and plays a major role in the loss of mechanical properties of the stent. We have developed a system which preserves – as much as possible – this molecular weight throughout the various steps of stent preparation. These issues have been solved as well, by taking into account the coefficient of hydration, thus inducing a memory of self-expansion based on the polymer properties. We therefore developed a proprietary moulding process, a proprietary memory shaping process, an exclusive designed crimping technology, resulting in preserving the molecular weight at > 85% of its initial weight from granules to sterilisation (Figures 1 and 2).


Figure 1. A. PLA stent gently crimped on the balloon resulting in neither strut cracks nor crazing; B. PLA expanded stent deployment presenting neither strut cracks nor crazing.
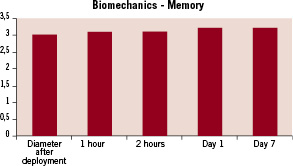
Figure 2. Graph showing the learning process of the PLA stent resulting in a slight, 5% increase of the stent diameter (Y axis, in mm) one hour after in vitro deployment, and 10% after 2 hours of in vitro deployment. This results, without recoil, at 7 days.
Bioresorption
Bioresorption of the polymer plays a critical role since it interferes with the duration of the mechanical properties of the stent: the biocompatibility of its degradation products, the coefficient of poly-dispersity, hydration. There is no clear answer with regards to the optimal duration of the bioresorption process which takes in account the duration of the scaffolding. Bioresorption is a major property of the polymer stents, and it is also classically considered as a potential threat because of the inflammatory reaction that may be induced. Van der Giessen et al have studied various bioresorbable polymer platforms, showing the issue of the lack of biocompatibility during the resorption period20. We evaluated in vitro and in vivo resorption, via the changes in molecular weight and the hydration. It appears that our in vitro model follows precisely the in vivo model (Figures 3 and 4). Based on these data, and pending the choice of the polymer, it is possible to anticipate the level of bioresorption at a precise time-point. This will impact on the control of the molecular weight at all steps before the use of the stent itself, and during its in vivo implantation. The bioproducts of degradation will also play a critical role in biocompatibility; indeed, the theoretical degradation of polylactic acid in lactic acid monomers results in H2O and CO2. However, depending on the quality of the synthesis and the transformation process, the degradation may result in the creation of incomplete degradation by-products (oligomers) that are highly proinflammatory through their crystalline forms. This situation is enhanced by the fact that the artery wall undergoes, at the same time, a healing process including a local inflammatory reaction caused by the presence of macrophages. During the resorption phase, our stent is well embedded by the healing process itself, thereby preventing any late migration of a dismantling strut.
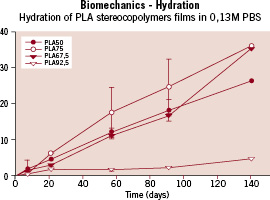
Figure 3. Graph showing the evolution of the hydration of various PLA stereoco-polymer films (Y axis: percentage of hydration) throughout time (X axis: time in days).
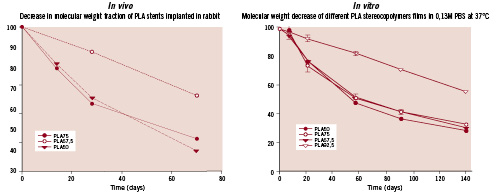
Figure 4. Graphs showing the evolution of the resorption of various PLA stereoco-polymer films in vitro (right) and explanted PLA stents in vivo (left) (Y axis: percentage of molecular weight) at various time-points (X axis: time in days). Comparison of the 2 graphs demonstrates the good relationship between our in vitro and in vivo models.
Biocompatibility
Biocompatibility represents the ultimate issue which influences the mid- and long-term outcome of the stent20. It is related by biodegradation as shown in past studies; to such a degree, that several programs of bioresorbable stents have considered it mandatory to include a drug elution system to prevent neointimal hyperplasia related to inflammation. It is also related to biomechanics in a positive manner, since the mechanico-genic stimulus induced by the radial force disappears when the degradation of the stent reaches the stage of dismantling. This represents, by itself, a major advantage of the bioresorbable stent over the permanent stent. In order to optimise the biocompatibility of polymers, we evaluated bioresorbable polymers issued from different sources, from variable D/L ratios. This resulted in differences in smooth muscle cell proliferation and macrophage accumulation, especially around the struts. The differences were particularly discernible at four weeks, whereas at 10 weeks the differences were no longer significant. In terms of endothelial coverage and collagen accumulation, there was no difference; PLA was recovered independently of the origin and the specificity. Our work then focused on the influence of the various steps altering the polymer variability upon biocompatibility. This was to optimise the choice of the polymer with regard to biocompatibility, which is, to our knowledge the critical issue. Our choice has been a consensus between biocompatibility, bioresorption, and biomechanical issues. Our current product has been tested in vitro and in vivo at one, four and six months in a rabbit model. This model was chosen because it represents the most appropriate response to compare in humans with regards to thrombosis, inflammation, and healing. In contrast to other groups, we aimed at evaluating the biocompatibility of our stent without the influence of drug elution. Our stent showed a remarkable ability to be deployed without recoil and strut partial or total breakage. At one month, endothelialisation was 100% completed, inflammation, smooth muscle cell proliferation and collagen accumulation was no more than after balloon angioplasty (Figure 5). At six months, the stent was completely integrated in the artery wall, thus preventing any strut migration secondary to the bioresorption process. Immunohistochemistry shows that the peak PLA resorption did not result in an increase or even persistence of inflammation, as has been reported with other bioresorbable polymer stents (Figure 6). Moreover, inflammation and smooth muscle cell proliferation were almost not detectable, resulting in a stable status translating into no hyperplasia, i.e., no in-stent restenosis. This result is important, since it justifies our strategy, dedicated to coping with the healing process without the need of drugs, thus preventing stent thrombosis or late malapposition, as shown in Figure 7. Last but not least, at six months there was no endothelial dysfunction (Figure 8).
In brief, bioresorption of the well synthesised and processed PLA can occur with excellent biocompatibility, and biomechanics, without preventive need of drugs. It can therefore be compared to a good wine, which can be digested without provoking a headache, thereby without necessitating the preventive absorption of Tylenol.
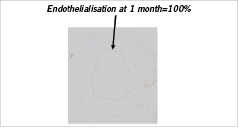
Figure 5. CD 31 immuno-marquage of a stented iliac rabbit artery showing complete endothelialisation of the stent one month after in vivo deployment.

Figure 6. RAM 11 immuno-marquage of stented iliac rabbit arteries at 1, 4, and 6 months showing almost no macrophage infiltration at early stage, translating into absence of inflammation and an excellent biocompatibility despite PLA resorption at 6 months.
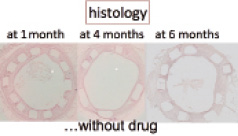
Figure 7. Hemalin eosin staining of stented iliac rabbit arteries at 1, 4, and 6 months with well embedded stents showing a satisfactory healing process without neointimal hyperplasia, despite PLA resorption.
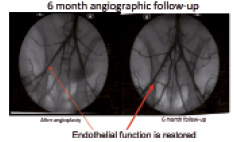
Figure 8. Angiograms of stented iliac rabbit arteries immediately and 6 months after deployment; exhibiting at 6 months a lack of endothelial dysfunction despite PLA resorption.

