
Abstract
To overcome the limitations of metallic stents, the development of the bioresorbable vascular scaffold started about 30 years ago. Researchers anticipated a transformative revolution from “vascular reparative therapy” by BRS at the beginning of its development. To date, there are five commercially available bioresorbable scaffolds which have already gained CE mark. However, recent studies, including randomised trials and meta-analyses evaluating clinical results of BRS, have raised concerns about the safety and efficacy of the device in the first few years prior to its complete bioresorption, compared to contemporary metallic DES. As one of the efforts to address these concerns, the impact of implantation technique was investigated. In addition, there are several aspects to be improved such as mechanical integrity, strut configuration, and late structural discontinuity. Intensive researches into the underlying causes of the greater device thrombosis rates with BRS have stimulated improvement of implantation technique and the development of next-generation BRS. Just as we have witnessed the evolution from first- to second-generation metallic DES, we anticipate that future generations of BRS with thinner struts and enhanced mechanical properties will result in substantially improved intermediate-term outcomes and safety.
Introduction
For the treatment of coronary artery disease, a metallic stent is a permanent approach to a condition that needs a temporary solution – namely vessel scaffolding and prevention of recoil, constrictive remodelling and restenosis, all of which occur within one year or less. The presence of a permanent metal cage may have numerous deleterious long-term effects including vessel straightening, loss of compliance, vasoregulation and adaptive remodelling, and the potential for late inflammation and mechanical failure. Many interventional cardiologists have long wondered whether it would be possible to scaffold a vessel transiently without using a permanent metallic implant to keep the vessel largely patent. Once the risk of restenosis has abated, the scaffold should disappear, restoring the vessel to as close to its native condition as possible.
The potential and theoretical clinical benefits of bioresorbable scaffolds (BRS) over current metallic stent technology can be summarised as follows: 1) reduction in long-term adverse events stemming from permanent materials1,2; 2) feasibility of non-invasive imaging, such as computed tomographic angiography or magnetic resonance imaging3; 3) maintaining suitability for future possible treatment options (either percutaneous or surgical) in multivessel disease, bifurcations, and long lesions; and 4) implantation in ST-segment elevation myocardial infarction patients (frequently young patients, with less extensive disease)4.
Fully bioresorbable scaffolds have been designed to realise the tenets of vascular reparative therapy: renewed compliance, dynamic vasomotion and mechanotransduction. Healthy compliance of the vessel can be progressively restored5. The full disappearance of the struts –which has been documented by ultrasound, OCT, histology and pharmacologically induced dynamic vasomotion– suggests that the vessel wall will once again sense the mechanical strains of pulsatile blood flow (cyclic pulsatility), which is an important stimulus for the cell biology of the vessel wall5. As blood is pumped through the coronary vessels, the vessel wall is exposed to two sets of forces, shear stress and cyclic strain. The interplay of shear stress and cyclic strain controls cell signalling, which can lead to atheroprotective/thromboresistant changes or disease progression and instability (mechanotransduction). The return of cyclic pulsatility and mechanotransduction may be of paramount importance in effecting optimal repair of the vessel wall.
In this review, celebrating 40 years of PCI, we summarise the history of bioresorbable scaffolds from their early development to their future potential.
Past
DEVELOPMENT OF BRS
Research efforts to create BRS started about 30 years ago. It was at that time that Richard Stack was working on a biodegradable stent at Duke University. Patrick Serruys met him at the American Heart Association in 1988 where they shared their interest in developing a new field of bioresorbable stenting. It was only later that it became evident that this would be a very complex and difficult endeavour, and manufacturers and major device companies showed little interest in the development of biodegradable technologies. Wim van der Giessen and Patrick Serruys continued working on the concept of developing a stent with a biostable polymer. The results of this scaffold implantation in the coronaries of a pig model were satisfactory and were published in the Journal of Interventional Cardiology in 19926. In 1996, the biocompatibility of synthetic polymers was investigated in porcine coronary arteries using Wiktor stents coated with five different types of biodegradable polymer (polyglycolic acid/polylactic acid copolymer, polycaprolactone, polyhydroxy-butyrate/-valerate copolymer, polyorthoester, and polyethyleneoxide/polybutylene terephthalate). The result showed marked inflammation leading to neointimal hyperplasia and/or thrombus formation7. Subsequently, Lincoff et al demonstrated that, in a porcine model, a stent coated with high-molecular-weight (321 kDa) poly-L-lactic acid (PLLA) was well tolerated and effective, whereas a stent coated with low-molecular-weight (80 kDa) PLLA was associated with an intense inflammatory neointimal response8. They also demonstrated the feasibility of drug elution (dexamethasone) from the PLLA. In 1998, Yamawaki et al reported that, in the porcine model, the fully biodegradable PLLA stent with tyrosine kinase inhibitor efficiently suppressed restenotic changes9. These pioneering experiments with high-molecular-weight PLLA further supported investigations in humans. However, despite the impressive results of these early stents, the technology failed to develop, primarily because of an inability to manufacture an ideal polymer that could limit inflammation and restenosis, and secondarily because of the growing interest in metallic drug-eluting stents (DES).
IGAKI-TAMAI STENT
The Igaki-Tamai PLLA coronary stent was the first fully bioresorbable stent to be implanted in humans. The first-in-man study demonstrated no major adverse cardiac events (MACE) or stent thrombosis (ST) within 30 days and only one repeat percutaneous coronary intervention (PCI) at six-month follow-up. Encouragingly, the Igaki-Tamai BRS did not induce an excess of intimal hyperplasia compared to bare metal stents (BMS). Furthermore, intravascular ultrasound (IVUS) imaging demonstrated no significant stent recoil at day 1, and continued stent expansion in the first three months after implantation10. At the 10-year clinical follow-up, freedom from all-cause death, cardiac death, and MACE was 87%, 98%, and 50%, respectively11. In the limited cases with serial angiographic follow-up, the minimum lumen diameter was stable. Despite these impressive results, the failure of the stent to progress was related primarily to the use of heat to induce self-expansion. There were concerns that this could cause necrosis of the arterial wall, leading to excessive intimal hyperplasia or increased platelet adhesion, leading to ST12. This polymer-only device also lacked incorporation of an antiproliferative drug. Subsequently, efforts to develop BRS continued, most of the data available stemming from the Absorb™ BVS (Abbott Vascular, Santa Clara, CA, USA).
ABSORB BVS 1.0 AND FIRST-IN-MAN ABSORB COHORT A TRIAL
The bioresorbable vascular scaffold (BVS) 1.0 design had a polymer backbone of PLLA coated with a thin layer of a 1:1 mixture of an amorphous matrix of Poly-D,L (racemic)-lactic acid (PDLLA) polymer, and 100 µg/cm2 of the antiproliferative drug everolimus. Physically, the scaffold has struts with a thickness of 150 μm and a crossing profile 1.2 mm, and consists of circumferential out-of-phase zigzag hoops linked together by three longitudinal struts between each hoop. It needs to be stored at -20°C to prevent creep, physical ageing of the polymer and to ensure device stability13. The first live case of Absorb implantation was transmitted from the Erasmus Medical Center at CRT 2006 (Moving image 1).
The BVS 1.0 design was tested in the first-in-man ABSORB cohort A study which enrolled 30 patients. At six-month follow-up, the angiographic in-stent late loss was 0.44 mm, mainly due to a mild reduction of the stent area (-11.8%) as measured by IVUS (chronic recoil). The neointimal area was small (0.30 mm2), with a minimal area obstruction of 5.5%, demonstrating effective suppression of restenosis by everolimus14. The fast bioresorption process allowed early loss of mechanical support and subsequent constrictive remodelling. To enhance the mechanical strength of the struts and to reduce early and late recoil, the strut design and the manufacturing process of the polymer were modified in the revised version, Absorb 1.1.
Present
ABSORB COHORT B
The second-generation Absorb BVS (1.1 design) was studied in the ABSORB cohort B study in 101 patients. The patients were divided into two different serial imaging follow-ups: cohort B1 at six and 24 months; cohort B2 at 12 and 36 months. The first six-month assessment showed that the modified manufacturing process of the polymer and geometric changes in the polymer platform substantially improved the medium-term performance (in-device late loss of 0.19±0.18 mm) of the scaffold15. In the 12-month cohort, the in-device late lumen loss (LLL) was 0.27±0.32 mm, pharmacological vasomotion of the scaffold vessel was restored, and most importantly there was no scaffold area loss16. Serial observation at six months and two years showed that in-device LLL increased from 0.16±0.18 mm to 0.27±0.20 mm (p<0.005), whereas mean scaffold area increased from 6.42±1.17 to 7.08±1.73 mm² (p<0.001). The MACE rate was 6.8% without any scaffold thrombosis17. The three-year follow-up showed stable luminal dimensions with an in-device late loss of 0.29±0.43 mm and a MACE rate of 10% without any scaffold thrombosis18. The five-year follow-up confirmed these results. When patients with a target lesion revascularisation were included (the worst scenario), the in-stent late loss was 0.32±0.48 mm19. At five years, struts were no longer discernible by OCT or IVUS. The overall five-year MACE rate was 11% without any thrombotic event. Only one event (a TLR) occurred after three years, the time of complete bioresorption.
ABSORB II
Following the encouraging results of ABSORB cohort B, we concluded that the performance of the second-generation Absorb BVS justified a randomised trial, with the best-in-class metallic drug-eluting stent as a comparator. The first patient was randomised in the ABSORB II trial in November 201120. The co-primary endpoints of ABSORB II were superiority in vasomotion and non-inferiority in angiographic late lumen loss of the Absorb drug-eluting bioresorbable scaffold at three years when compared with the XIENCE® metallic DES (Abbott Vascular). Quantitative differences in vasomotion were not observed between the devices, and late loss in the Absorb BVS was significantly larger than in the XIENCE stent21. Whether the lack of difference in vasomotion between the devices may have been due to the angiographic technique of assessment or the sole use of nitroglycerine as a vasodilator requires further study.
The device-oriented composite endpoint (cardiac death, target vessel myocardial infarction [TV-MI], clinically indicated target lesion revascularisation) at three years was higher in Absorb than XIENCE (10% vs. 5%, p=0.425), although the event rates observed in ABSORB cohort B were considered acceptable in the absence of comparators. In addition, there were nine cases of definite/probable scaffold thrombosis in Absorb, whereas no stent thrombosis was observed in XIENCE (p=0.0331). Those safety signals have led to a detailed examination of the optimal technique required to implant Absorb, and to potential iterations in device design that might improve outcomes, as discussed later22-26.
MAGNESIUM-BASED BIORESORBABLE SCAFFOLD
In contrast to polymer-based BRS, Magmaris™ (Biotronik AG, Bülach, Switzerland) is made of a refined, slower-degradable magnesium alloy and has a modified electropolished strut cross-sectional profile to slow down resorption and to prevent fracture27. As part of the inherent nature of metal, magnesium scaffolds offer high tensile strength which can potentially offer good compliance of the scaffold without fracture during scaffold deployment28.
The bioresorbable magnesium scaffold without drug elution was tested in the first-in-man PROGRESS study, in which 63 patients with a single de novo lesion were treated with 71 scaffolds. There was a high incidence of TLR (45%) at 12 months and a relatively high LLL on angiography performed at four-month follow-up (1.08 ± 0.49 mm). Vasomotor function was assessed in five treated segments at this time point and appeared restored29. Design changes were made to slow the degradation of the scaffold to prevent chronic recoil.
DREAMS-1 (Biotronik AG), the iteration preceding Magmaris, was a paclitaxel-eluting scaffold made of a magnesium alloy. The BIOSOLVE-I trial enrolled 46 patients with 47 lesions at five European centres30. At three-year follow-up, three target lesion failures had occurred (6.6%), consisting of two clinically driven target lesion revascularisations that were performed at scheduled six-month angiography (4.3%) and one myocardial infarction after drug-eluting balloon treatment in a non-target lesion in a non-target vessel that occurred at 12-month angiography (2.2%). No cardiac deaths or scaffold thrombosis (ScT) occurred31.
Subsequently, Magmaris, a sirolimus-eluting magnesium scaffold, was assessed in the prospective, multicentre, first-in-man BIOSOLVE-II trial (N=123)32. In-scaffold LLL was 0.39±0.27 mm at 12-month follow-up. Target lesion failure occurred in four patients (3.4%), consisting of one death, one target vessel myocardial infarction and two clinically driven target lesion revascularisations. During the entire 12-month follow-up, none of the patients experienced a definite or probable ScT27. Long-term clinical outcomes have not been reported.
CURRENT BIORESORBABLE SCAFFOLDS
As of May 2017, five BRS – Absorb, DESolve® (Elixir Corp., Milpitas, CA, USA), ART Pure (Arterial Remodeling Technologies, Noisy le Roi, France), Fantom® (REVA Medical, San Diego, CA, USA), and Magmaris – have acquired the CE mark (Table 1). The Absorb scaffold has also been approved by the Food and Drug Administration (FDA) in the USA and by the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan. Among the CE-marked scaffolds, the Absorb device is the only scaffold with randomised evidence.
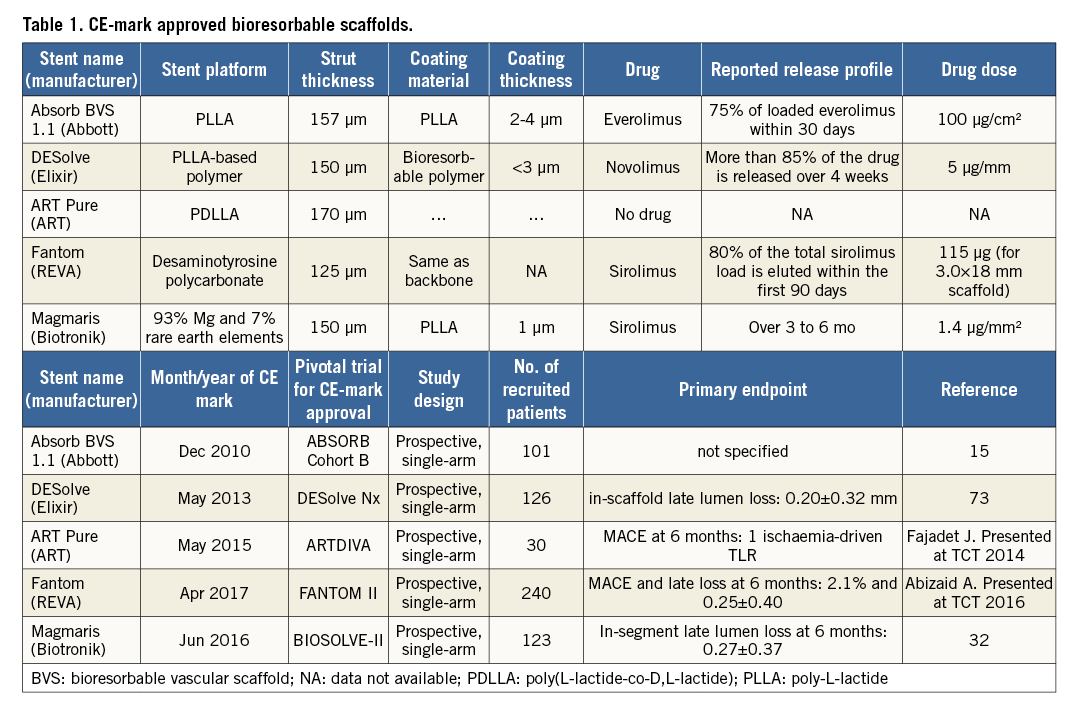
Current BRS are composed of either a polymer or a bioresorbable metallic alloy. Numerous different polymers are available, each with different chemical composition, mechanical properties, and consequently bioabsorption times (Figure 1). The most frequently used polymer in the current generation of BRS is PLLA. The key mechanical traits for candidate material in coronary indications include high-elastic moduli to impart radial stiffness, large-break strains to impart the ability to withstand deformations from the crimped to expanded states and low-yield strains to reduce the amount of recoil and overinflation necessary to achieve a target deployment.
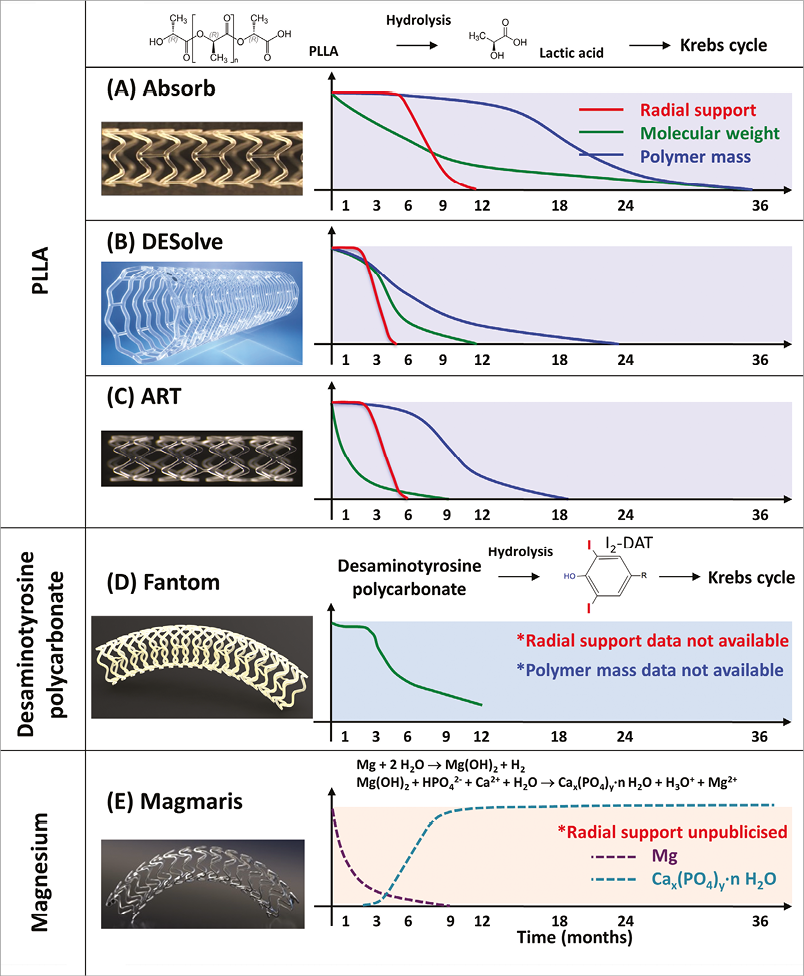
Figure 1. Biodegradation process of CE-mark approved bioresorbable scaffolds. (A) Absorb41,66, (B) DESolve67,68, (C) ART, (D) Fantom48,69, and (E) Magmaris70. (modified from Sotomi et al71).
It was anticipated that ScT in the very late phase after DES implantation would be solved with the advent of fully bioresorbable scaffolds. However, recent long-term follow-up data of Absorb from randomised trials21,33-35 and observational studies36 show the worrisome signal of a higher thrombotic risk in the very late period (>1 year) as well as before one year. A total of seven randomised trials have been completed comparing the Absorb BVS to XIENCE. In a meta-analysis by Collet et al25, including five trials with at least 24 months of follow-up, Absorb BVS had a higher risk of definite/probable device thrombosis compared with XIENCE EES (OR 2.93, 95% CI: 1.37-6.26, p=0.01), whereas the difference in target lesion failure (TLF) was not significant. Mahmoud et al37 and Sorrentino et al38 with two-year results of AIDA and ABSORB III included, suggested significantly higher rates of TLF, as well as higher rates of definite/probable device thrombosis in Absorb BVS than in XIENCE EES. The meta-analysis by Montone et al39 confirmed significantly higher rates of ST and TLF, with the finding that BVS had a higher risk of subacute, late, and very late ST, whereas the risk of TLF and TLR was higher between one and two years with no difference in the first year. Finally, the most recent two-year meta-analysis of the seven randomised trials by Ali et al also demonstrated that BVS was associated with higher rates of composite device-oriented adverse events and device thrombosis cumulatively at two years (Figure 2) and between one- and two-year follow-up compared to everolimus-eluting stents (EES)40. Comparison of these six meta-analyses is presented in Table 2 and Figure 3. The latter meta-analysis also included an individual patient-level pooled analysis from ABSORB II, ABSORB Japan, ABSORB China, and ABSORB III, demonstrating that, compared to metallic EES, BVS had higher two-year rates of TLF, driven by an increase in the rates of TV-MI and device thrombosis with BVS during the one-year to two-year follow-up (Figure 4). Theoretically, a period of two to three years is still too short to assess the real value and the potential benefit of PLLA-based BRS, since the biodegradation and biointegration processes take >3 years to be completed. Preclinical studies in a porcine coronary model with intracoronary imaging analysis demonstrated that biodegradation is completed at approximately three years and followed by biointegration that is completed at three to four years41,42. Therefore, very long-term follow-up for up to 10 years may be required to draw scientific conclusions on this theoretical advantage. However, given the greater risk of thrombosis with Absorb BVS in the short term or midterm, optimal scaffold implantation and prolonged dual antiplatelet therapy should be carefully considered in patients treated with this device to ensure that the early safety profile is comparable to contemporary metallic DES.
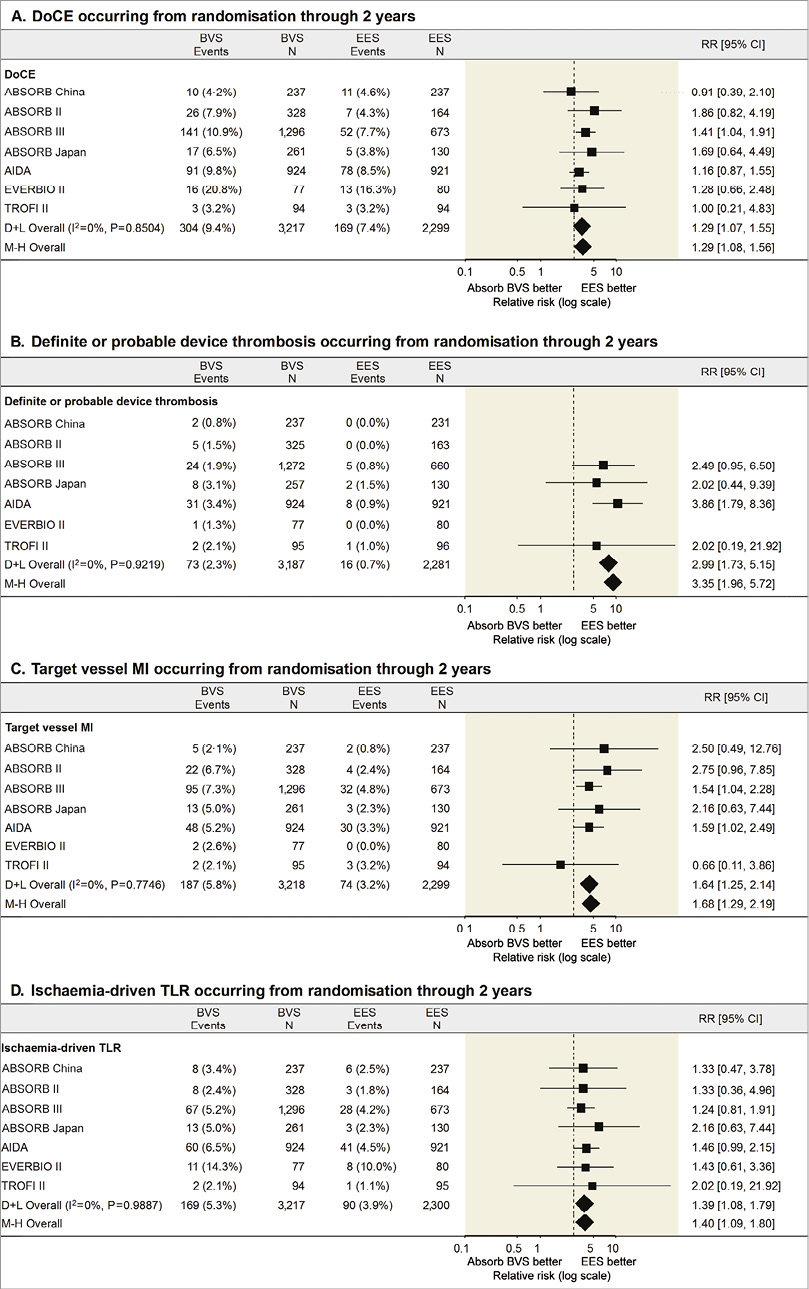
Figure 2. Two-year comparison of the Absorb BVS vs. EES for selected clinical outcomes from seven randomised trials40. A) The device-oriented composite endpoint (DoCE) of target lesion failure (cardiac death, target vessel myocardial infarction, or ischaemia-driven target lesion revascularisation). B) Device thrombosis (definite or probable). C) Target vessel myocardial infarction. D) Ischaemia-driven target lesion revascularisation. CI: confidence interval; D+L: DerSimonian and Laird random effects model; M-H: Mantel-Haenszel fixed effect model; RR: risk ratio
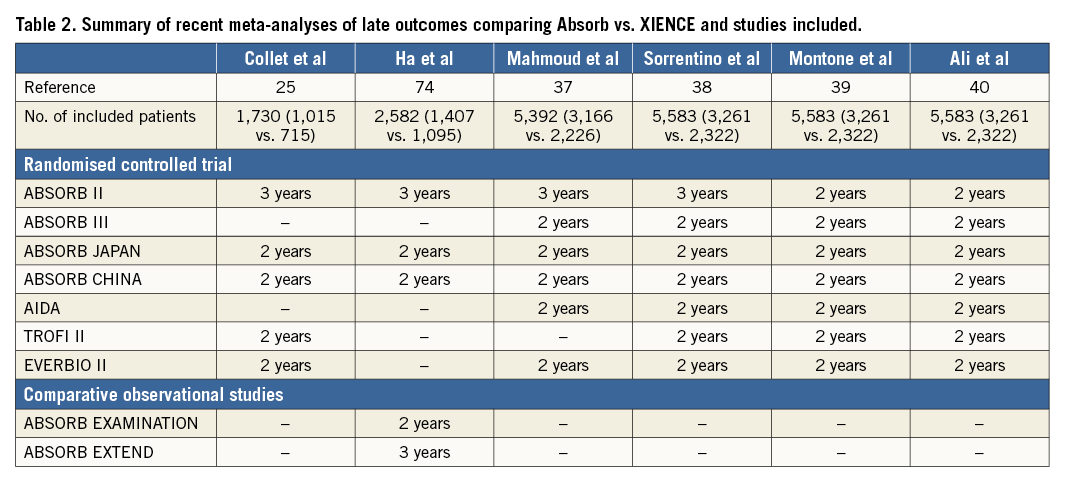
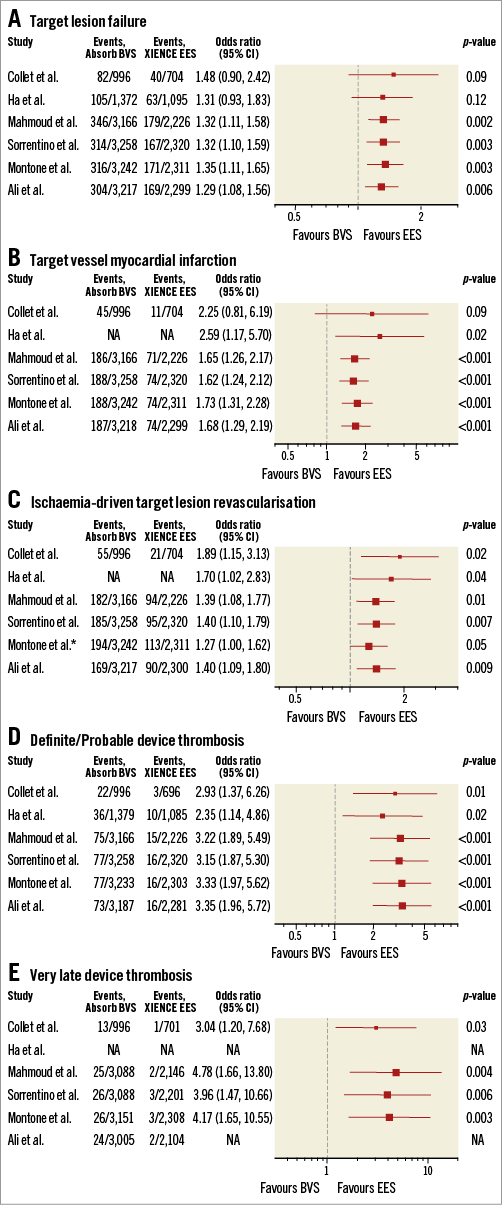
Figure 3. Forest plot of recent meta-analyses of late outcomes comparing Absorb vs. XIENCE. A) Target lesion failure. B) Target vessel myocardial infarction. C) Ischaemia-driven target lesion revascularisation. D) Definite/Probable device thrombosis. E) Very late device thrombosis. Odds ratios from each study are shown. Studies included in each meta-analysis are summarised in Table 2. *Any TLR. BVS: bioresorbable vascular scaffold; CI: confidence interval; EES: everolimus-eluting stent; NA: not available
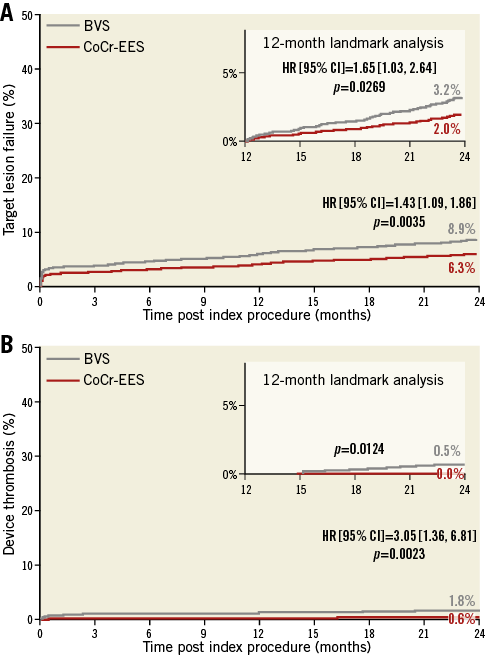
Figure 4. Two-year and one- to two-year cumulative time-to-first-event curves for patients randomised to the Absorb BVS vs. XIENCE CoCr-EES from the four randomised ABSORB trials. A) The device-oriented composite endpoint (DoCE) of target lesion failure (cardiac death, target vessel myocardial infarction, or ischaemia-driven target lesion revascularisation). B) Device thrombosis (definite or probable). BVS: bioresorbable vascular scaffold; CoCr: cobalt-chromium; EES: everolimus-eluting stent. Reprinted with permission from The Lancet40.
THE IMPACT OF DEVICE SIZING AND IMPLANTATION TECHNIQUE
The investigators of the ABSORB II and ABSORB EXTEND trials pooled their patients and thereby revealed the fact that a mismatch between vessel size (too small) and device size (too large) documented by Dmax could create an abnormal density of polymer in the lumen and result in an early incidence of periprocedural MI43. The impact of mismatch on late events was not evident at three-year follow-up in the ABSORB II trial (Figure 5).
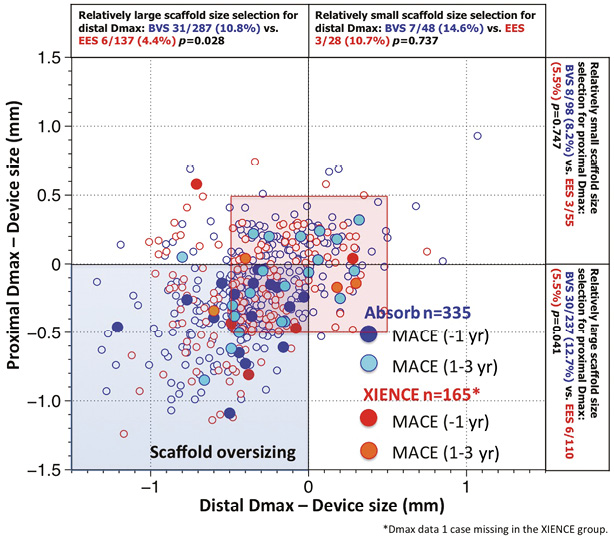
Figure 5. Major adverse cardiac events (MACE) in the first year and at one to three years as a function of mismatch between device and vessel size. During the first year of follow-up of ABSORB II, MACE occurred exclusively in patients/lesions (dark blue filled circles) in which the scaffold was oversized with respect to the vessel diameter (nominal size of the scaffold larger than both Dmax proximal and distal, left lower quadrant). Over the next two years, MACE (light blue filled circles), including six scaffold thromboses causing STEMI and TLR, were no longer clustered exclusively in the quadrant corresponding to oversized scaffold, but the late MACE events at three years were distributed in the four quadrants and situated, for the most part, in the red frame defining lesions that had received a nominal size scaffold within the range of 0.5 mm with respect to the proximal and distal Dmax43. Distal Dmax: maximal diameter distal to the lesion; Proximal Dmax: maximal diameter proximal to the lesion
A specific implantation technique for BRS was first introduced by Puricel et al, and has come to be known as PSP: preparation, sizing, and post-dilatation. They have implemented, in their routine practice, a specific technique of implantation44, consisting of the following components: i) predilatation with a non-compliant balloon up to the same size as the reference vessel diameter (RVD); ii) BRS implantation only in case of full expansion of the non-compliant coronary angioplasty balloon as demonstrated by angiography in two orthogonal planes; iii) implantation of a BRS of the same size as the RVD at 10 to 12 atm; iv) post-dilation with non-compliant balloons up to a maximum of 0.5 mm larger than the nominal scaffold diameter at 14 to 16 atm. Although the study was retrospective, the optimised implantation strategy demonstrated a lower incidence of scaffold thrombosis (1.0%) as compared to the “early experience (without specific protocol)” group (3.3%). Tanaka et al also reported acceptable event rates in a very complex all-comers population: 11.6% of TLF and 1.2% of definite/probable scaffold thrombosis at two years when the optimised implantation strategy was utilised, with no thrombosis events after one year45. Interestingly, intravascular imaging was performed in the majority of cases in this series (85.8%), demonstrating the need for further intervention in 24.5% of lesions even after routine post-dilatation, suggesting the importance of intravascular imaging. Subsequently, Ortega-Paz et al investigated the predictive value of PSP scores on clinical outcomes in the GHOST-EU registry46. The performance of PSP was shown to be an independent predictor of a reduction in the device-oriented composite endpoint (DoCE). The univariate analysis of the six very late scaffold thromboses in ABSORB II has potentially identified one IVUS parameter, i.e., expansion index <0.6 (p<0.001), that is suspected of being involved in the late occurrence of a sudden scaffold thrombosis.
Current limitations
MECHANICAL INTEGRITY
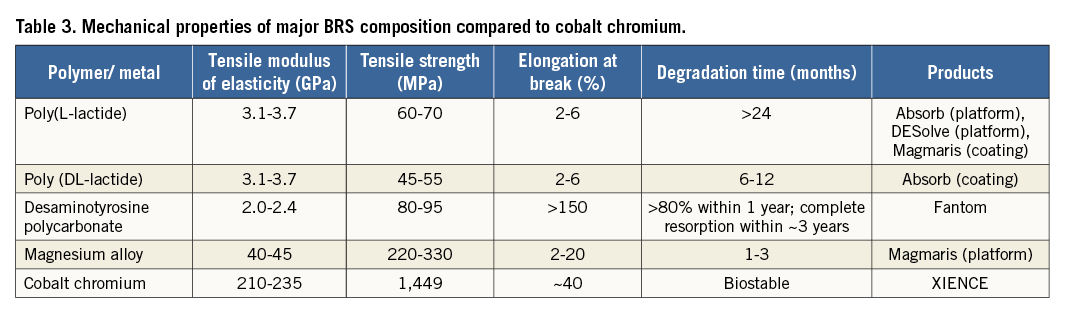
If a bioresorbable scaffold is ultimately expected to have similar applicability to a durable metal stent, the gap in mechanical properties must be reduced. Currently, three primary limitations exist: 1) low tensile strength and stiffness which require thick struts to prevent acute recoil, 2) insufficient ductility which limits the range of scaffold expansion during deployment, and 3) instability of mechanical properties and late structural discontinuity during dismantling (Figure 6)47. Table 3 shows that polylactide has a tensile strength ranging between 45 and 70 MPa and has very low elongation at break between 2 and 6%. Desaminotyrosine polycarbonate, of which the most recent CE-approved BRS, Fantom, is comprised, has relatively high elongation at break of >150%, with a substantial expansion safety margin (~1.0 mm depending upon device diameter)48. Magnesium already has a much better tensile strength up to 300 MPa with elongation at break of 20%. To place these findings in perspective, current cobalt-chromium DES have a tensile strength of 1,500 MPa with an elongation at break of 40%.
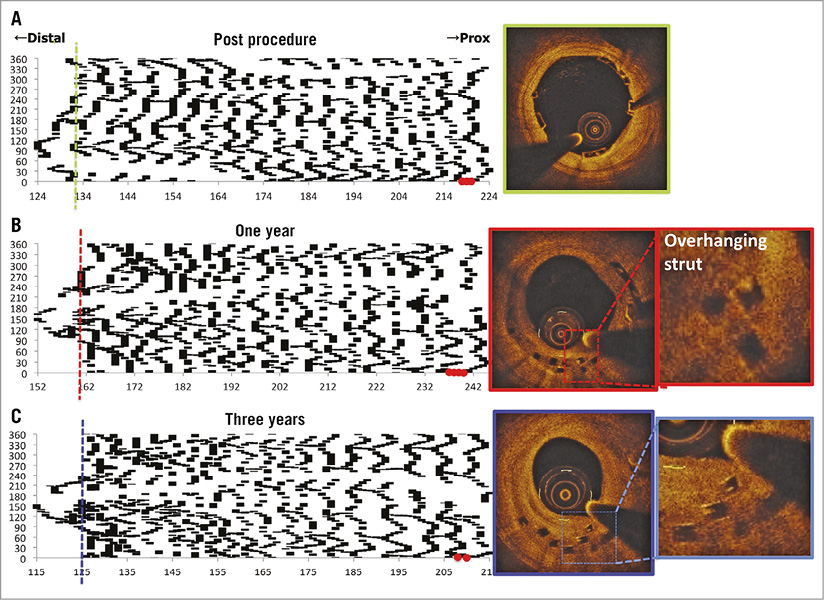
Figure 6. Serial assessment of late discontinuities using spread-out-vessel graphics. A)-C) The foldout views represent spread-out-vessel graphics created by correlating the longitudinal distance from the distal scaffold edge to the individual struts detected in a single cross-section (abscissa) with, on the ordinate, the angle where the individual strut was located in the circular cross-section with respect to the centre of gravity of the vessel (ordinates). In each cross-section (axial resolution of 200 µm), the circumferential length of each individual strut was depicted in an angular fashion. The resultant graphic represented the scaffolded vessel, as if it had been cut longitudinally along the reference angle and spread out on a flat surface. The spread-out view post procedure (A) showed that the scaffold consisted of 19 rings interconnected by three links. At one year (B) and three years (C), mechanical integrity has gradually subsided and the distal part of the scaffold was starting to show signs of dismantling, along which late discontinuities were observed. At baseline, in the distal edge of the scaffold (green dotted line in the foldout view), two-dimensional optical coherence tomography (OCT) (green frame) revealed well-apposed struts. At one year, in the distal edge (red dotted line in the foldout view), two-dimensional OCT (red frame) showed overhung and apposed struts. At three years, these struts remained overhung (blue line in the foldout view, corresponding to two-dimensional OCT with a blue frame). The phenomenon is considered benign because the struts are mostly covered at one and three years. Red dots represent the proximal metallic markers47.
STRUT CONFIGURATION
Stent developers look to increase stent strut dimensions to compensate for the mechanical shortcomings of bioresorbable materials. The first generation of BRS had relatively thick struts. As the thickness of these struts increases, strain levels imposed on the material increase proportionally. However, disturbed endothelial shear stress and platelet activation from thick struts could constitute a nidus for thrombus (Figure 7)49. Large strut thickness induces subsequent fibrin deposition50, which may cause restenosis. Current quadratic thick struts with wide footprints are difficult to embed (Figure 8), and the tissue composition of the vessel wall (fibrotic, calcified) may further preclude embedment of the large footprint of the Absorb struts (Figure 9)51. As a consequence, cellular coverage of the polymeric material is delayed. Moreover, greater strut thickness leads to the device having a larger profile, resulting in more difficulty in delivering the device through tortuous and non-compliant arteries as compared to slimmer metallic comparators (despite inherently greater longitudinal flexibility of the polymer compared to metal). Ongoing efforts promise to reduce strut thickness while maintaining radial force by changing polymer composition, processing and scaffold design.
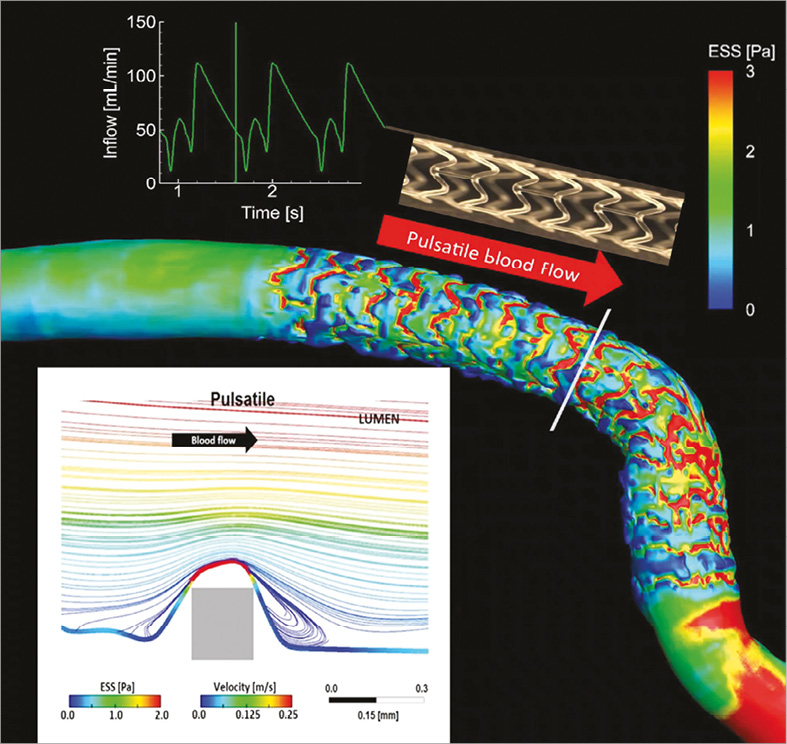
Figure 7. Pulsatile shear stress following scaffold implantation. Shear stress in a human coronary artery scaffolded with the Absorb BRS. Moving image 2 demonstrates the pulsatile shear stress simulations of the same case throughout a cardiac cycle. To calculate the computational fluid dynamics, angiography has been fused with OCT. Shear stress was computed assuming pulsatile flow and non-Newtonian fluid to depict the shear stress in systole and diastole. A colour barcode depicts the shear stress values in pascal (Pa) units. In early systole, the scaffolded coronary artery is almost uniformly blue due to very low shear stress. Conversely, in early diastole at the time of high flow velocity, the scaffolded area is globally red with a shear stress around 3 Pa. It should be noted that the struts of the Absorb platform are easily recognisable on this video and it should also be emphasised that in diastole there is high shear stress (red) on top of the struts and low shear stress (blue) distal and proximal to the struts with signs of reversal of the flow at the foot of the struts, as demonstrated by the local streamlines shown in the excerpt (upper right panel in Moving image 2). In Moving image 2, the two lower panels show colour-coded fly-through views of the baseline situation (lower left panel) immediately after implantation and five years later (lower right panel). Initially, the corrugated appearance of the endoluminal surface is evident with the presence of indigo colour on the top of the struts and dark blue colour at the bottom of the struts in regions of very low shear stress. At five-year follow-up, the corrugated appearance due to the strut protrusion has disappeared and regions of low shear stress in dark blue are almost non-existent in the scaffolded area which is, on the contrary, characterised by an alternation of green and red colour which corresponds to a more physiological shear stress (1-3 Pa).
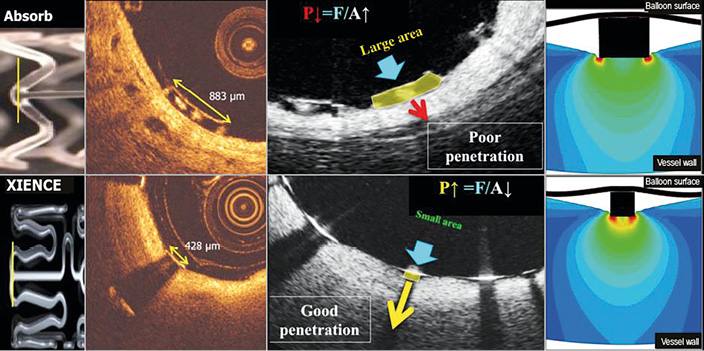
Figure 8. Footprint of the strut and embedment. The upper row represents OCT cross-sections of the Absorb BRS scaffold while the lower row depicts the cross-section of the XIENCE stent. The vertical lines (yellow) superimposed on both devices in the left side panel correspond to OCT cross-sections at ψ-hinge (psi-hinge) level. The ψ-hinges are the distal part of a longitudinal connector, where the angle between the connector and the W-shaped ring is acute. The ψ-hinge strut width (yellow two-sided arrows) of the Absorb scaffold can reach up to 883 µm while the strut width of the XIENCE stent is only 428 µm. When the same balloon pressure is applied to the large footprint of Absorb (middle, upper row) and the small footprint of a metallic strut (middle, lower row), the metallic strut (like an ice-skate in snow) can be embedded and expanded by the dilating balloon much better than with the Absorb device (like a snowshoe in snow)72.
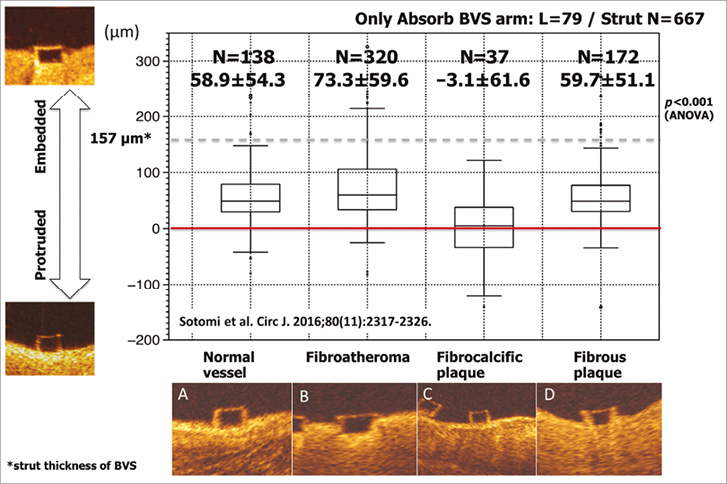
Figure 9. Correlation between embedment depth and plaque morphology. Embedment depth has been assessed according to different plaque morphologies: normal vessel (A), fibroatheroma (B), fibrocalcific plaque (C), and fibrous plaque (D). The best embedment is observed in fibroatheroma. Data are shown as box-and-whisker plots and mean±standard deviation51.
LATE STRUCTURAL DISCONTINUITY (DISMANTLING)
During the bulk erosion process, discontinuities naturally develop in the scaffold. If not constrained by neointima, protrusion of resorbing scaffold elements into the lumen may result in very late scaffold thrombosis. Theoretically, this phenomenon can be minimised by optimal technique ensuring lack of malapposition at the time of implantation. The optimal duration of the bioresorption process (ranging from three to 42 months depending on the polymer) is unknown and is being evaluated in preclinical and clinical studies42,52-54.
Future
DEVICE IMPROVEMENT
The refinement of scaffolds with thinner struts while preserving strong radial force is considered necessary and is ongoing. Newer-generation devices are aiming at thinner struts with a smaller crossing profile compared to the currently available versions of the bioresorbable devices (although strut width will remain greater than with metallic DES). Tensile strength and radial force can be increased by altering the molecular orientation of PLLA. Through a heating and extrusion process, undrawn semicrystalline polymer can become oriented and stronger structures created55. For example, the Mirage sirolimus-eluting Bioresorbable Microfiber Scaffold (Mirage BRMS; Manli Cardiology Ltd., Singapore) is a scaffold with a PDLLA backbone. The struts of the Mirage are circular in shape with a thickness of 125 μm in scaffolds with a diameter ≤3 mm (Mirage-125), and 150 μm in scaffolds with a diameter ≥3.5 mm (Mirage-150). An animal study which compared strut embedment and endothelial shear stress between Mirage BRMS and Absorb BVS showed favourable results in the Mirage BRMS56. Specifically, it demonstrated less protrusion and higher mean shear stress in scaffolded segments of the Mirage BRMS as compared to the Absorb BVS. However, in a randomised trial comparing Mirage and Absorb, in-scaffold late loss at 12 months did not show statistical difference57.
Among CE-marked BRSs, Fantom has the thinnest struts (125 µm), and is novel in that the iodinated polymer backbone is radiopaque. Next-generation products that are not yet CE-marked include the Fortitude® sirolimus-eluting BRS with 150 μm struts58, Aptitude® with 115 μm and Magnitude® with <100 μm struts (all Amaranth Medical, Mountain View, CA, USA). These devices are composed of an ultra-high amorphous molecular weight PLLA which maintains radial strength while providing 1.5 mm or more overexpansion capability. Other devices include the MeRes100™ sirolimus-eluting BRS with 100 μm struts (Meril Life Sciences Pvt. Ltd., Vapi, India)59, and the Firesorb™ sirolimus-eluting BRS with 100-125 μm strut thickness (Shanghai MicroPort Medical, Shanghai, China). These second-generation BRSs offer the potential for substantially improved clinical outcomes compared to first-generation devices.
For adequate evaluation, these future scaffolds should be studied in randomised controlled trials versus contemporary metallic DES, which is the responsibility of clinical investigators, physicians, and the industry.
BVS-SPECIFIC PROCEDURES
Although the PSP strategy is widely recognised in the community of interventional cardiologists, its actual efficacy has not yet been demonstrated due to the lack of prospective randomised trials, which are unlikely to be logistically or ethically feasible. New scientific insights regarding the PSP strategy are emerging. For example, long-term expansive remodelling might be triggered by greater initial barotrauma as quantified by the expected balloon to artery ratio greater than 1.25 60. Finally, operator experience – with or without the discipline of a PSP strategy, with or without the guidance of OCT45 – may impact on the short-term and long-term clinical results of BRS. This was the case with the BMS and drug-eluting stent in the SCAAR registry that initially reported an excess of mortality and myocardial infarction with the DES in the early phase of recruitment, whereas the outcome was reversed in favour of DES when the operators became more experienced with the technique and optimal patient and lesion selection61-63.
ANTIPLATELET THERAPY IN BRS
For metallic DES, a six-month duration of dual antiplatelet therapy (DAPT) after PCI for stable ischaemic heart disease is recommended in European and American guidelines, and 12 months is recommended in acute coronary syndromes64,65. The optimal duration of DAPT for BRS remains to be investigated. Nonetheless, current clinical results suggest a need for a longer duration of DAPT at least until the complete biodegradation of the devices. Many investigators believe it is prudent to continue DAPT for up to three years after BRS implantation in patients not at high risk for bleeding. However, these recommendations would be level of evidence “C” due to the lack of randomised data and studies specifically designed to address the optimal duration of DAPT after BVS implantation. Among such studies, BVS LATE is planned to randomise 2,000 patients to aspirin and clopidogrel dual therapy or clopidogrel monotherapy at 12 months after BVS implantation (NCT02939872).
Conclusions
Although researchers anticipated a transformative revolution from “vascular reparative therapy” by BRS at the beginning of its development, recent studies, including randomised trials and meta-analyses evaluating clinical results of BRS, have raised concerns about the safety and efficacy of the device in the first few years prior to its complete bioresorption, compared to contemporary metallic DES. Intensive researches into the underlying causes of the greater device thrombosis rates with BRS have stimulated improvement of implantation technique and the development of next-generation BRS.
Authors’ perspective
Just as we have witnessed the evolution from first- to second-generation metallic DES, we anticipate that future generations of BRS with thinner struts and enhanced mechanical properties will result in substantially improved intermediate-term outcomes and safety. Ongoing adequately powered trials with follow-up to 10 years (ABSORB IV, clinicaltrials.gov identifier: NCT02173379) will determine whether BRS improve long-term outcomes compared to metallic DES.
Conflict of interest statement
G. Stone reports personal consultant fees from St. Jude, Toray, Matrizyme, Ablative Solutions, Claret, Sirtex, V-wave, Vascular Dynamics, Miracor, Neovasc, Medical Development Technologies, BackBeat Medical, Valfix, TherOx, and REVA, and reports equity in Qool Therapeutics, Caliber, Aria, Biostar family of funds, MedFocus family of funds, Guided Delivery Systems, Micardia, and Cagent; Columbia University receives royalties from Abbott Vascular for the sale of the MitraClip. Y. Onuma has served as a member of the advisory board for Abbott Vascular and has received speaker honoraria from Terumo. P.W. Serruys has served as a member of the advisory board for Abbott Vascular. The other author has no conflicts of interest to declare.
Supplementary data
Moving image 1. The first live case of Absorb implantation at Erasmus Medical Center (CRT 2006).
Moving image 2. Pulsatile shear stress following scaffold implantation.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. The first live case of Absorb implantation at Erasmus Medical Center (CRT 2006).
Moving image 2. Pulsatile shear stress following scaffold implantation.

