KEYWORDS
Abstract
Vascular stents have revolutionised the field of interventional cardiology. Once an artery has healed, however, stents are no longer thought to serve a functional role. Bioabsorbable stents would be preferred to permanent implants if they maintain arterial architecture, minimise device/host interactions, and reduce the need for long-term anticoagulation therapy. Technical challenges to develop and commercialise a successful bioabsorbable stent relate to identification of materials and stent designs capable of balancing acute and chronic mechanical properties, degradation time, and biocompatibility. Successful programs will be ones that achieve these requirements with uncompromised product deliverability, efficacy and safety. Many materials currently proposed for use in bioabsorbable stents take longer than 24 months to degrade and so may not meet these criteria. We describe here the Medtronic CardioVascular bioabsorbable program which focuses on developing a degradable stent for superficial femoral arteries that targets degradation in less than 12 months.
Abbreviations
ACC: American College of Cardiology
AHA: American Heart Association
BMS: bare metal stent
DAPT: dual anti-platelet therapy
DES: drug-eluting stent
MLD: minimum lumen diameter
TLR: target lesion revascularisation
Introduction
Since its inception in the late 1970s interventional cardiology has undergone a number of major paradigm shifts where the standard of care has transferred from percutaneous transuminal balloon angioplasty to implantation of bare metal (BMS) and drug-eluting stents (DES)1. These revolutionary changes have typically occurred every 10-15 years. DES quickly became the treatment of choice following the launch of the first sirolimus releasing stent (Cypher; Cordis Corporation, Bridgewater, NJ, USA) in 2001. As we enter the second decade of the twenty-first century, the obvious question is ‘What is the next major innovation after DES?’
For years, bioabsorbable stents have been one of the most popular answers to this question. While stents provided a solution to dissections and vessel recoil following angioplasty procedures, they are generally thought to serve little function once healing of the artery has taken place. A stent that ‘does its job and then goes away’ would return a vessel to a more natural state and facilitate re-intervention should access to the lesion area be necessary in the future. It would also support a wider variety of imaging modalities such as MRI. Furthermore, the concept of a temporary medical implant is an attractive concept for many patients, especially as procedures on younger patient populations become more and more common. (Table 1)

Clearly, all things being equal, bioabsorbable devices will be preferred to permanent implants. Unfortunately, all things are not equal, and it will be necessary to make some compromises in device performance, ease of use, and maybe even take on additional risks in the interim in order to achieve a bioabsorbable stent platform. This article presents a number of technical challenges that have been encountered in Medtronic’s bioabsorbable stent program as we strive to understand what trade-offs might be acceptable and transfer the postulated advantages into reality.
Selecting the appropriate target indication
Coronary artery disease
The coronary stent opportunity is very attractive from a marketing standpoint with over $5 billion in annual revenue worldwide2. It is a well penetrated and established market, especially in developed countries where reimbursement is available. While not perfect, BMS and DES devices function relatively well as the standard of care for all but the most complicated situations such as diffuse disease, anatomical challenges and other considerations.
Unmet coronary needs from a clinical perspective include providing consistent and predictable patient outcomes to curtail target lesion revascularisation (TLR) procedures while reducing the risks of very late stent thrombosis. Current guidelines (ACC/AHA) recommend dual antiplatelet therapy (DAPT) be maintained for 12 months (in the absence of bleeding risk)3, although the authors admit this decision was not based on specific trial experience, but more because of concerns of stent thrombosis rates in trials requiring only six months DAPT4. Yet, minimising long-term DAPT is a target need due to cost burdens, challenges with managing patient compliance, and the serious outcomes associated with DAPT interruption due to unplanned surgeries. The latter is a serious issue, which may affect 20-30% of patients in the first year after stenting and drives much of BMS use. Thus, one of the central requirements for any new coronary stent system, including a bioabsorbable stent, should be to minimise the duration of DAPT treatment.
Peripheral artery disease
Vascular disease in the popliteal and superficial femoral arteries (SFA) represents a smaller but non-trivial and growing market opportunity with over $750 million in projected annual worldwide sales by 20135. Here, vessel calcification and high stresses due to complex vascular motion have contributed to stent fracture and elevated restenosis rates in most devices. As no single treatment approach exists, the SFA market is highly fragmented with dozens of companies, large and small, vying for their share of the market.
Medical treatment in the SFA is often performed progressively moving from balloon angioplasty to debulking to stenting in an effort to maintain treatment options. Bioabsorbable technologies have potential here to bestow more durable outcomes while still facilitating opportunities for subsequent procedures should they be needed. Furthermore, since SFA therapies are typically life-enhancing rather than life-sustaining, the regulatory paths and risks associated with developing an unproven technology are greatly reduced compared to coronary indications.
Due to the unmet clinical need and growth opportunities in the periphery, Medtronic has decided to focus bioabsorbable development efforts on an SFA stent solution while striving to understand and monitor opportunities related to coronary indications.
Properties of an ideal stent
Any successful bioabsorbable stent will need to be safe, deliverable, and effective compared with current therapies. Safety will be related to biocompatibility of the material and degradant products before, during, and following degradation. An appropriate rate of degradation of twelve months or less is also critical to achieve suitable antiplatelet therapy duration and facilitate patient follow-up. Deliverability should be comparable with biostable stent alternatives, and require minimal deviations to standard catheterisation laboratory practices regarding handling. Efficacy targets should likewise be similar to current equivalent products with respect to TLR and major adverse cardiac event (MACE) rates. Additionally, stent materials should possess acute and chronic mechanical properties to impart radial strength for sufficient durations of time prior to significant degradation taking place.
Selecting the appropriate material
The primary factor in determining the success or failure of a bioabsorbable stent program is going to be the selection of an appropriate material. The two main families of candidates are absorbable metals such as magnesium-based alloys and polymer-based materials susceptible to hydrolytic breakdown. Within the polymer-based material family one can select from ‘traditional’ poly-α-hydroxyesters, such as polylactide, polyglycolide, poly-ε-caprolactone, polytrimethylene carbonate, and polydioxanone, which have been utilised for over thirty years in the suture and orthopedic fields6. New polymer chemistries based on tyrosine-derived polycarbonate, salicylate-based polyanhydrides, and polyurethane-based chemistries have also been explored in an attempt to improve mechanical performance and degrade into benign or even therapeutic breakdown products. Additional polymer materials can also be created through blending or copolymerisation to further design specific attributes of the material.
The challenge is to select a material that balances the trade-offs with respect to:
– Mechanical properties and stent design: must maintain adequate device performance with respect to radial strength and deliverability.
– Degradation rates and mechanical properties: must provide scaffolding for sufficient duration to allow healing.
– Degradation rates and biocompatibility/safety: must disappear in a time-frame that provides patient benefit without overloading the system with inflammatory by-products as it degrades.
Mechanical properties
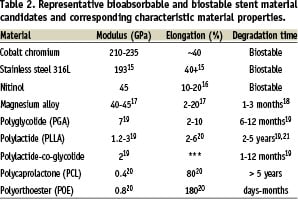
Compared to cobalt chromium, stainless steel, and other materials that are currently being used in stent fabrication, bioabsorbable candidates are substantially inferior from a mechanical strength perspective. Summarised in Table 2, polymer materials typically exhibit a 100-fold lower elastic modulus while magnesium-based alloys are approximately 5-fold lower. Since elastic modulus is directly proportional to radial stiffness, stents made from these materials would need to possess 240% and 50% thicker struts, respectively to maintain equivalent performance. These differences are illustrated in Figure 2. Consequently, material processing and stent design modifications are needed to maximise the performance of bioabsorbable materials.
Polymer processing
Processing can be used to orient individual polymer chains and strengthen the material. Similarly, increases in molecular weight, or the average lengths of the polymer chains, can also improve mechanical properties – especially toughness and elongation prior to failure. While these effects can be substantial (e.g., two to three fold increases in modulus with chain orientation), the resulting properties are still inferior to those of current stent materials. Initially, increasing orientation facilitates motion between polymer chains and generates an increase in the elongation possible prior to break. This process, however, reaches a maximum where additional orientation reduces break strain and may result in strut fractures (Figure 1A).
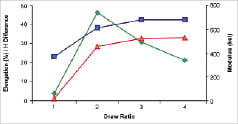
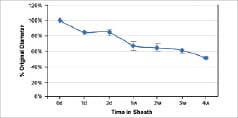
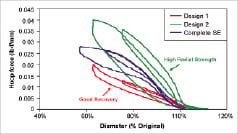
Figure 1. Polymer chain orientation, crush recovery and relaxation. A) The orientation, as well as average length and average molecular weight can affect mechanical properties. B) Self-expanding stent recovery properties. C) Post-deployment relaxation properties.
Types of processing
In general, polymers can be processed through extrusion, moulding and casting. To manufacture a polymer part like stent, extrusion is the most suitable processing methods. During extrusion, the polymers are heated to above its flow temperature and extruded out through a die to form desired shapes. Further orientation of the polymer chains can be achieved after or during the extrusion process to gain improved properties as stated above. However, due to the reactive nature of the bioabsorbable polymers, the polymers will start to break down at high temperature, which then limits the extent of property improvement through chain orientation.
The effects of polymer processing result from the act of placing the material in a metastable state. The energy used to orient the material also generates an entropic driving force to return the original condition. The regression can take place quickly at elevated temperatures such as those encountered in ethylene oxide sterilisation or more slowly over the course of weeks to months at ambient and physiologic temperatures. This creates a challenge for shelf-life and storage as the product may be continually changing over time.
Coronary applications
Bioabsorbable coronary stents are associated with crossing profile, radial strength, and stent fracture challenges. The key mechanical traits for a successful candidate material in coronary indications include high elastic moduli to impart radial stiffness; large break strains to impart the ability to withstand deformations from the crimped to expanded states; and low yield strains to reduce the amount of recoil and over-inflation necessary to achieve a target deployment. Challenges with elastic moduli were discussed previously.
Stent fracture during deployment is a serious challenge with bioabsorbable coronary designs. Material candidates with highest moduli also tend to have lower break strains. For example, poly-L-lactide (PLLA) and WE43 magnesium based alloys have break strains of 1-5% and 2%, respectively compared to cobalt chromium MP35N which can be deformed to 40%7. See additional properties in Table 2. A cobalt chromium stent (3.0 mm Driver; Medtronic CardioVascular, Santa Rosa, CA, USA) coronary stent may experience as much as 14% percent strain at the crown during deployment. The low break strains typically found in magnesium or polymeric materials leave bioabsorbable stents subject to crown fracture.
Stent developers look to increase stent strut dimensions to compensate for mechanical shortcomings of bioabsorbable materials. As the thickness of these struts increases, strain levels imposed on a material increase proportionally (Figure 2). Lastly, many bioabsorbable coronary designs employ large opening angles between struts as circumferentially oriented support segments further offset strength limitations. In addition to limiting the range of undersizing and oversizing available to match dimensions of a target vessel, the increased deformation to achieve these large angles will contribute to stent fracture at the crowns.
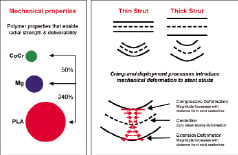
Figure 2. Stent mechanical properties. A) Relative comparisons between polylactides (PLA), cobalt chromium (CoCr) and magnesium (Mg) mechanical properties. B) Effect of crimping and deployment dynamics on mechanical deformation.
Stent fractures will lead to premature radial strength loss, vessel recoil, tissue irritation, and inflammation independent of the material biocompatibility. As discussed earlier, break strains can be manipulated to some extent via processing, but it remains to be seen whether processing and material selection can offset these demands with an adequate safety window as design modifications push these strain requirements higher.
Peripheral applications
Self-expanding delivery approaches are considered standard of care in the SFA. Key mechanical challenges for bioabsorbable SFA candidates include adequate opening force, dimensional recovery, and reproducible performance under multiple loading cycles. The overwhelming numbers of peripheral self-expanding devices are fabricated using nitinol, which is rather unique in that it exhibits super-elasticity which permits reversible deformation up to 8% strain. Comparatively, polymer candidates typically have recoverable strain limits in the 3-4% range. This coupled with lower elastic moduli results in designs that either fully expand but have inferior radial strength or are relatively strong but lack dimensional recovery (Figure 1B).
Radial strength is further complicated by differences in loading and unloading behaviour. A number of bioabsorbable design prototypes are capable of opposing crush during loading test cycles, but they typically generate minimal outward force during unloading cycles resulting in a device that sits in the vessel with little ability to push plaque against the vessel wall. This could restrict widespread use of bioabsorbable stents to non-calcified lesions and situations where pre-dilation achieves adequate results.
While the mechanical loading environment on devices in the SFA is notoriously demanding, no single loading cycle is as demanding as the transition from the crimped to uncrimped configuration. Typically, permanent diameter changes of 5-15% or more are observed. Braided designs exhibit better opening properties (Figure 1C) but lack to radial strengths of patterned tube designs made from the same materials. Balloon-based or balloon-assisted delivery strategies have also been considered but are challenging because of damage associated with expanding stents beyond the point of recoverable deformation to induce a dimensional change. Alternative strategies, including curing in situ, present theoretical solutions to deployment challenges but have been difficult to implement with reliable consistency.
Viscoelastic mechanical properties
For polymer candidates, their viscoelastic properties make reproducible performance of an SFA stent under multiple loading and unloading cycles a challenge. Because of their long chain nature, the response of polymer materials to foreign force or deformation is slow and time dependent. After several loading cycles, this lag of response builds up and causes drop of opening force and recoil of the stent. For a self-expanding stent, the ability to gradually self-expand is lost over time.
The effects of polymer viscoelasticity can affect stent function both pre-implant during storage and post-implant as negative remodelling forces act over time. Changes in the dimensional recovery have been observed in braided self-expanding stents held in the crimped state (Figure 1C). Devices that reopened completely immediately following crimping only recovered 84% of their intended diameter after 24 hours in a delivery sheath, and by week four this recovery had dropped to 51%. Balloon expandable devices encounter the reverse problem as storage leads to loss of stent retention.
Once implanted, water absorption and physiologic temperatures increase viscoelastic challenges. The effects of viscoelasticity were likely evident in the prospective, open-label ABSORB trial where 3.0-mm BVS stents (Abbott Vascular, Santa Clara, CA, USA) exhibited a drop in minimum lumen diameter (MLD) from 2.32±0.31 mm following the procedure to 1.89±0.31 mm at six months as assessed via quantitative coronary angiograph8. This loss was attributed to a late recoil effect as little neointimal hyperplasia was observed due to the presence of everolimus and the stent area was shown to decrease 11.8% over the first six months after implantation.
Degradation rates
The mechanisms by which bioabsorbable materials break down and are resorbed by the body present some additional challenges to material selection. While all the macromolecules in a polymer are fabricated from the same repeating monomer subunit-or subunits in the case of a co-polymer. The number of subunits and consequently the length of the chain will vary from molecule to molecule.
Following implantation, water penetrates into the polymer matrix where it interacts with chemical groups along the backbone to cleave the polymer chain into two fragments. This process continues until the chains reach a point where physical disentanglement occurs (Figure 3). At this point the stent loses much of its mechanical integrity, but the polymers are still too long to be resorbed. Hydrolysis continues generating more and more chain fragments until eventually a point is reached where the chain fragments can be made soluble or actively absorbed through macrophages.
Varying aspects such as initial molecular weight and degree of crystallinity can influence the point where mechanical integrity is lost but will not appreciably alter the duration between this point and polymer resorption.
In the case of polylactide, mechanical integrity disappears at 6-9 months while complete mass loss does not take place for 24-60 months9. Since degradation and clearance of polymers are active and potentially pro-inflammatory processes, DAPT may need to be administered throughout the entire degradation process. This will extend the duration of critical post-procedural surveillance and anti-thrombotic therapy beyond current label indications.
While the exact duration of required mechanical strength for adequate healing is unknown, a material that disappears too quickly also presents challenges. The absorbable magnesium stent (AMS; Biotronik, Berlin, Germany) has been shown to disappear within two months in animal studies10. The PROGRESS-AMS trial, a randomised, prospective study, evaluated 71 stents (3.0 and 3.5 mm diameter, 10 and 15 mm length) implanted into predilated de novo coronary lesions. At four months, the MLD had dropped from 2.18±0.38 mm post-stenting to 1.34±0.49 mm. In-stent neointimal hyperplasia was responsible for 41% of the lumen loss while negative remodelling/recoil and extra-stent tissue growth accounted for an additional 42% and 13.5%, respectively. Additionally, 25 of the 63 patients (39.7%) required target lesion revascularisation (TLR) within the first four months. Of these revascularisations, 15 (23.8%) were ischaemia-driven. The rapid material resorption and subsequent early loss of radial strength was identified as a major contributing factor for lumen loss.
When the results of PROGRESS-AMS are considered in conjunction with the late recoil diameter loss observed in the BVS ABSORB trial8, one can begin to define 3-6 month targets for the minimum duration required for mechanical scaffolding. We have worked with a number of material candidates, including polyurethanes and polylactide-based co-polymers in an attempt to target a 12 month degradation time. It remains to be seen whether it will be possible to achieve a 3-6 month radial strength with these materials.
Shorter degradation rates will support manageable durations for patient monitoring and facilitate commercialisation paths in what is likely to be a lengthy and expensive regulatory process. Tracking patients post-procedurally to provide proper follow-up and ensure antiplatelet therapy compliance can already be a challenging undertaking but will only become worse if stent degradation lasts 24 months or more. Similarly, clinical trials that require substantial monitoring post-degradation prior to device and reimbursement approvals make the technology less attractive to develop as resorption times increase.
Biocompatibility profile
Successful bioabsorbable candidates must remain biocompatible before, during, and following degradation. Bioabsorbable materials may be comparable to standard conventional devices in the acute post-procedural time and may provide improvements after complete resorption has occurred (Figure 3). The risk however, is what happens in the interim period where active degradation is taking place. Safety during degradation will be paramount to a successful product, and if the majority of mass loss does not occur for 18-24 months, then the largest safety risk could be years following the procedure.
While it is attractive to think that polymers like PLLA will degrade via the Kreb cycle to carbon dioxide and water, the reality is that degradant products that will be exposed to and resorbed by the vascular tissue are likely to be moderately-sized oligomers (3000-7000 Daltons) of the starting material. Hydrolysis of many bioabsorbable polymers, including lactide and glycolide-based chemistries results in the formation of carboxylic acid and alcohol groups for every bond cleaved. This build up of end groups can lead to local drops in pH (Figure 3). If too many of these groups form too quickly, the surrounding tissue is unable to manage the build-up acidity and inflammation results. While the geometries of stent struts may be thin enough to facilitate transport of buffering elements such that a build up of acidic by-products within the core does not lead to accelerated degradation as has been reported with larger implants such as bone screws11. It is difficult to measure local pH changes and comprehend resulting effects on tissue proximal to stent struts.
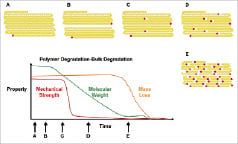
Figure 3. Polymer degradation. A) Long chain monomer units absorb water, followed by B) cleavage at random location to generate two chains. C) Chains reach point of physical disentanglement resulting in loss of mechanical strength. D) As hydrolysis continues, the concentration of acidic end groups increases such that E), the chains become short enough to become soluble and actively resorbed by inflammatory cells.
Biostable polymers used in first generation commercialised DES coatings have been shown to elevate tissue inflammation and have been associated with delayed healing and late stent thrombosis12. While it may be possible to avoid these responses through the use of coating polymers designed to improve biocompatibility13,14, there are fewer options for alternative chemistries in bioabsorbable polymers. Furthermore, the quantity of material in a stent will present a substantially greater bio-burden to the tissue. For example while an 18 mm coronary DES typically may contain less than 0.5 mg of polymer to control elution; the stent itself would contain ~25 mg of material. In peripheral SFA stents where device lengths are frequently 100 mm or more, the amount of material can exceed 750 mg representing a 1500 times increase in degradant products exposed to the tissue.
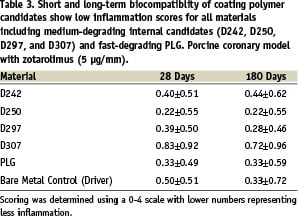
Use of anti-proliferative drugs may also be able to offset potential tissue responses assuming the degradation takes place while the drug is still present. We have observed that relatively quickly degrading materials such as PLG and internal lactide-based polymers developed by Medtronic for DES coating, have exhibited low inflammation when evaluated as coating materials in combination with zotarolimus using a porcine coronary model. Inflammation at 28 (early degradation) and 180 days (active or post-degradation) is summarised in Table 3.
It remains to be seen how bioabsorbable polymers may affect tissue when implanted into an atherosclerotic and potentially pro-inflammatory environment. Introducing injury and inflammation in the presence of high lipids has been shown to stimulate foam cell accumulation and atherosclerotic development. We have observed high levels of inflammation associated with the degradation of endoluminal coverings and have used these gels to create focal lesions in a hypercholesterolaemic rabbit model. Bioabsorbable implants likely to exhibit the best biocompatibility and safety profiles will be the ones that possess small quantities of material and distribute degradation products over sufficient intervals such that the surrounding tissue can manage the burden.
‘Ease of use’ requirements
In order for bioabsorbable stents to be adopted, it will be important for them to be easily incorporated into standard catheterisation laboratory practices. Special storage requirements such as refrigeration or lengthy, complicated preconditioning steps before or during the implant procedure are likely to be met with resistance. This becomes important if the specific processing methods utilised to maximise performance of bioabsorbable materials lead to meta-stable states of organisation regress under elevated temperatures or storage intervals.
Visibility of the device under fluoroscopy has also been raised as a concern for accurate placement and follow-up. While the majority of bioabsorbable material candidates exhibit poor radiopacity, placement can typically be achieved via marker segments on the delivery system or incorporated into the stent. Furthermore, use of intravascular ultrasound and optical coherence tomography can also be employed as visualisation modalities.
What happens as the stent degrades?
A fundamental assumption behind the concept of a bioabsorbable stent is the idea that the device will degrade without negatively affecting the vessel. Clearly, insufficiently apposed struts and segments that span across vessel bifurcations may pose particulate risks as the material degrades. Similarly, rapidly degrading materials with insufficient endothelialisation may embolize prior to incorporation into the vessel wall. Perhaps more subtle though, is the impact of the artery’s natural response to changing haemodynamic stresses.
Under normal physiologic conditions, an artery will positively remodel to increase its diameter in response to chronic elevated shear stress. Correspondingly, thickening of the vessel wall takes place to react to sustained elevated blood pressure. One hypothesis suggests that using a stent to dilate an artery functions to activate many of the local stretch receptors similar to local hypertension. While the stent is present, the smooth muscle cells constrict against the device but are dominated by the opening force of the stent. Once the stent begins to break down, however, a point is reached where the constrict forces prevail over the opening force and a narrowing of the artery occurs. This suggests that it may not matter how long radial strength is maintained and that late recoil observed in the BVS ABSORB8 and AMS PROGRESS trials may inevitably take place with loss of mechanical strength10. On the other hand, this loss of diameter may be only transitory as the vessel could eventually readapt through positive remodelling once the stent is gone.
What is the future for bioabsorbables?
In light of the significant technical challenges, one may ask ‘is there a fit for bioabsorbable products in interventional cardiology?’ The answer lies in clearly addressing unmet clinical needs. In the case of coronary applications, the current DES products set a high performance standard while cost and complications of DAPT specify degradation targets of 12 months or less. Peripheral SFA applications appear to be the better product to target for development. Once safety can be proven in the periphery, it may then be possible to leverage that knowledge into other areas. Beyond coronary and SFA devices, non-load bearing indications such as coatings for DES, closure devices for large bore percutaneous systems and non stent-based drug delivery may represent additional opportunities for bioabsorbable technologies.
There is undoubtedly a lot of excitement about the promise of bioabsorbable stents in the research and clinical communities. Unfortunately, the hype does not always live up to expectations, and the market has gotten it wrong in the past. At one point, many were projecting brachytherapy as the next standard of care for vascular interventions. Similarly, many did not fully believe in the potential of DES until they took over the market. While there are clearly many challenges to address, the outcome and future of bioabsorbable stents remains to be determined.
Acknowledgements
The authors would like to thank Jane Moore, MS, for her assistance in the preparation of this manuscript.
Disclosures
Ms. Moore, like the authors of this article, is an employee and stockholder of Medtronic, Inc.

