Background
The permanent presence of an artificial implant is believed to be the potential trigger for late inflammation, which leads to restenosis and late stent thrombosis. Drug-eluting stents (DES) have effectively reduced the restenosis rate and the need for repeat revascularisation, but have raised concerns with regard to the risk of stent thrombosis, delayed endothelialisation and vessel vasoreactivity. New stenting technology has moved toward the development of temporary implants composed of biocompatible materials that mechanically support the vessel during the period of high risk for recoil and then completely degrade within 90-720 days. The motivation for the development of bioabsorbable stents was to restore vessel functionality and was driven by the need to solve the limitations of metallic stents, such as stent thrombosis, which requires prolonged antiplatelet therapy; mismatch of the stent to the vessel size, which often results in a smaller lumen after stent implantation; and artifacts with modern imaging technologies such as magnetic resonance imaging (MRI) and multislice computed tomography (MSCT).1 Bioabsorbable stents combine the mechanical prevention of vessel recoil with the advantages of long-term perspectives compared to permanent implants, including the possibility of late outward vessel remodelling and improved re-intervention options.2 This manuscript will focus on the design- and clinical development of the absorbable metallic (magnesium) stent (AMS).
Magnesium alloy composition, design evolution and future developments
The selection of a metallic alloy for bioabsorbable stents is intuitively attractive since it can provide similar radial strength with minimal acute recoil when compared to the currently used stainless steel and cobalt chromium metal stents. The magnesium alloy and its degradation products are biocompatible to the vessel wall with high tissue tolerance. Magnesium, even in high doses, has shown positive effects on the vascular system. As an essential element for the human body, magnesium is involved in the synthesis of more than 300 enzymes; it is known to cause vasodilation; to promote the development of collateral vessels in ischaemia; to directly inhibit acute stent thrombosis; and to have antiarrhythmic properties. The main challenge when using magnesium is its fragility, therefore, in order to add strength to the magnesium, other metal elements are added to the final composed tube: among these are different compositions of zirconium, yttrium, and other rare earth elements.3
Manipulation of the alloy and stent design can impact the degradation time; degradation rates vary from one to 12 months. The stent design mainly impacts radial force and time to stent fractures. Like polymeric bioabsorbable stents, the advantages of metallic bioabsorbable stents, when compared to conventional stents, are the elimination of late stent thrombosis, chronic inflammation, and artifacts during noninvasive imaging.4 Compatible with cardiac MRI and MSCT, these stents can be used as vehicles for possible drug and gene delivery.
The absorbable magnesium alloy developed by BIOTRONIK AG (Biotronik, Berlin, Germany) has undergone several iterations and has been refined over time. The first generation, AMS-1, was available in diameters of 3.0 and 3.5 mm and lengths of 10 and 15 mm. With a proprietary design and minimal recoil comparable to a metallic stent, they were implanted in human tibial and coronary arteries. The feasibility clinical trials detected fast degradation. Within a few weeks, the stent lost its strength and at three months the AMS-1 was completely degraded. While the requirements for scaffolding and radial force post-stent implantation have not been determined, the conclusion drawn from the first clinical trial was that the degradation should be slowed to maintain radial force. This discovery led to the development of the AMS-2.1, with several alloy and design iterations that resulted in the increase of radial force and a slower degradation rate as demonstrated on the bench and in animal studies. A radiograph demonstrating the differences in degradation between AMS-1 and AMS-2.1 is shown in Figure 1. AMS-2.1 serves as the stent platform for AMS-3, the drug-eluting AMS (DREAMS). Coating of the AMS-2.1, with a fast degradable material, results in a controlled elution of an anti-proliferative drug. Preclinical studies with AMS-3 have shown promising results at 28 days and show effective reduction in neointimal thickness compared to bare AMS. (Figure 2)


Figure 1. Radiograph demonstrating the differences between AMS-1 and AMS-2.1.
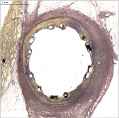

Figure 2. Demonstration of drug activity in a porcine coronary model at 28 days with the DREAMS prototype.
Absorbable metallic stents for paediatric application
Although the use of biodegradable metallic stents for paediatric applications is limited, they may play an important role in the treatment of congenital anomalies. In the first successful implantation of a biodegradable metal stent into the left pulmonary artery of a preterm baby, Zartner et al reported on the implantation of a biodegradable 3.0 mm magnesium stent performed in a hybrid procedure; and showed that reperfusion of the left lung was established and persisted throughout the 4-month follow-up period during which the gradual degradation process of the stent was completed. Despite the small size of the baby, the degradation process was clinically well tolerated. The mechanical and degradation characteristics of the magnesium stent proved to be adequate to secure reperfusion of the previously occluded left pulmonary artery.5 The infant died at seven months due to multiple organ failure. At autopsy, two months after the projected complete degradation time of the stent, no solid compounds were detected, and the vessel diameter had increased slightly to 3.7 mm. There was a low concentration of inflammatory cells; thin coverage with neointima; and there was evidence of cellular infiltration into former stent struts.6
Further, Schranz et al reported the first use of a bioabsorbable magnesium stent for acute treatment of a newborn with severely impaired heart function due to a long segment coarctation after a complex surgical repair. Extensive residual stenosis of descending aortic arch caused pulmonary hypertension with ventilator dependency. By angiography, the coarctation length was 13 mm. A 3.5x15-mm AMS inflated at 16 atmosphere was used and a final diameter of almost 4 mm was obtained. Re-catheterisation revealed a reduced diameter as a result of the ongoing stent degradation, therefore a second 4x15 mm magnesium stent was implanted. Despite the use of two metal stents, pathological magnesium levels in serum of the patient were not detected.7
Absorbable metallic stents for patients with chronic limb ischaemia
The bioabsorbable magnesium alloy stent developed by BIOTRONIK AG of Berlin, Germany, was the first of its kind for which it was proven that it could be implanted safely in infrapopliteal2,8,9 and human coronary arteries10-12 and that it was absorbed in the intended time frame. In a series of below-the-knee cases, Bosiers et al presented the first clinical results using AMS for treatment of infrapopliteal lesions in 20 patients with chronic limb ischaemia (CLI). At 12 months’ follow-up, angiographic procedural success, defined as 30% or less residual stenosis on visual assessment of the planned area of treatment after completion of treatment without serious adverse events and before the arterial sheath is removed, was achieved in all 20 patients. Post-procedural intravascular ultrasound (IVUS) control (performed in 19 patients) confirmed a good stent positioning, and a homogeneous and complete inflation of the AMS device in all investigated patients, as well as a low immediate elastic recoil comparable to conventional metal stents.9
Following the promising results of AMS in this compassionate use trial, the AMS INSIGHT (Bioabsorbable Metal Stent Investigation in Chronic Limb Ischaemia Treatment) trial was designed to compare, in a randomised controlled setting, the safety and efficacy of the implantation of a balloon-expandable absorbable stent in the infrapopliteal bed based on one- and six-month clinical follow-up and efficacy based on six-month angiographic patency. In the study, 117 patients with 149 lesions with CLI were randomised to implantation of an AMS (60 patients, 74 lesions) or stand-alone percutaneous transluminal angioplasty (PTA; 57 patients, 75 lesions). Seven PTA-group patients ‘’crossed over’’ to AMS stenting. The first-generation AMS-1 and the Pleon Explorer angioplasty balloon catheter (BIOTRONIK) were used. The primary safety endpoint was defined as absence of major amputation and/or death within 30 days after index intervention and the primary efficacy endpoint was the six-month angiographic patency rate as confirmed by core lab quantitative vessel analysis. The 30-day complication rate was 5.3% (3/57) and 5.0% (3/60) in patients randomised for PTA alone and PTA followed by AMS implantation, respectively. On an intention-to-treat basis, the six-month angiographic patency rate for lesions treated with AMS (31.8%) was significantly lower (p=0.013) than the rate for those treated with PTA (58.0%).13 Findings from AMS INSIGHT do not support the positive clinical outcome of the initial AMS findings in a small cohort of patients2,8,14 and indicate that the current-generation stent, although safe, does not meet the efficacy criteria.13
Absorbable metallic stents for human coronary arteries: lessons from the PROGRESS-AMS trial
The PROGRESS-AMS (Clinical Performance and Angiographic Results of Coronary Stenting) study was a prospective, multicentre clinical trial of 63 patients with coronary artery disease designed to address the safety and feasibility of AMS implantation in human coronary arteries. The primary endpoint was major adverse cardiac events at four months defined as cardiac death, nonfatal myocardial infarction, and ischaemia-driven target lesion revascularisation (TLR). Briefly, 71 stents, 10-15 mm in length and 3.0-3.5 mm in diameter, were successfully implanted after predilatation. Angiography and IVUS were conducted immediately after AMS deployment and at four months. The AMS was well-expanded upon deployment without immediate recoil. There were no adverse events including death, myocardial infarction and stent thrombosis in any of the patients enrolled. There were, however, high TLR rates in these patients at four months due to 27% restenosis. The major contributors for restenosis as detected by IVUS at four months were: decrease of external elastic membrane volume (42%), extra-stent neointima (13%), and intra-stent neointima (45%). The patients who restenosed responded well to repeat intervention with DES. Eight patients who did not require repeat revascularisation at four months underwent late angiographic and IVUS follow-up from 12 to 28 months. In these patients the long-term clinical angiographic and IVUS follow-up studies demonstrated durability of the results when compared with the four-month results. Paired IVUS analysis demonstrated complete stent degradation with durability of the results of the four-month IVUS indices. (Figure 3) The neointima was reduced by 3.6±5.2 mm3, with an increase in the stent cross sectional area of 0.5±1.0 mm2 (p=NS). The median in-stent minimal lumen diameter was increased from 1.87 to 2.17 mm at long-term follow-up. The median angiographic late loss was reduced from 0.62 to 0.40 mm by quantitative coronary angiography from four months to late follow-up.15 Long-term IVUS follow-up showing the disappearance of the stent strut and preservation of the vessel wall is shown in Figure 4.
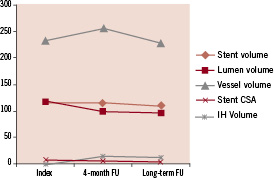
Figure 3. IVUS analysis.
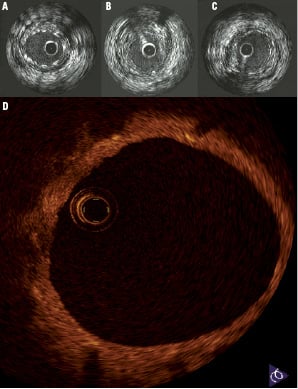
Figure 4. Long-term IVUS follow-up at A) Post-implantation; B) Four months; and C) Sixteen months; D) Optical coherence tomography of a human coronary vessel 15 months post-AMS implantation demonstrating complete healing of the vessel wall with complete disappearance of the stent struts.
As a substudy of PROGRESS-AMS, Ghimire et al aimed to investigate the endothelium independent coronary smooth muscle vasomotor function four months after implantation of the AMS (n=5) compared with a control group of patients implanted with permanent metal stents (PMS) (n=10) undergoing follow-up angiography, but who were free from angiographic restenosis. Quantitative coronary angiogram using an automated edge detection system was performed before and after the administration of 2 mg intracoronary isosorbide dinitrate. The vessel diameter was measured at 0.2-mm intervals throughout the stented segments and a 1 cm proximal reference segment. The cross sectional area was calculated before and after intracoronary isosorbide dinitrate administration, averaged, and the percentage change measured. Reference segments demonstrated preserved vasomotor function in all cases: +13.28% (AMS) versus +17.15% (PMS), p=0.39. The mean percentage increase in cross sectional area for the stented segment was +6.78% for the AMS versus –1.30% for PMS, p=0.003. In contrast to PMS, within the AMS-stented segments there is demonstrable vasodilatation.16
To conclude, clinical trial results suggest that implantation of the AMS-1 is safe in human coronary and peripheral arteries (150 patients). AMS-1 is associated with a high procedural success rate (99.4%), is well tolerated in a variety of vessels, and is CT/MRI compatible. The stent degrades as intended without adverse events or distal embolisation and the vessel regains its vasoreactivity properties. However, the implantation is associated with higher than expected restenosis rates, which are mainly contributed to early recoil and neointima formation.
Future generations of absorbable metallic stents
Two main objectives were targeted during the development of the next generations of AMS: prolonged mechanical stability and radial force and the further reduction of neointima hyperplasia. To improve upon AMS-1, significant changes were made to AMS-2.1, including a tailor-made specialty magnesium alloy with slow degradation, thinner strut thickness (120 µm compared to the 165 µm of the AMS-1), surface modifications, and a modern 6-crown design. Preclinical studies showed prolonged scaffolding and stent integrity, less neointima proliferation, and increased radial strength. The stent platform AMS-2.1 has diameters of 3.0, 3.25, and 3.5 mm and lengths of 12, 16, and 20 mm and favourable mechanical properties (for the 3-mm version), such as elastic recoil of 5%, a collapse pressure of 1.2 bar, a crossing profile of 1.3 mm, and 6 Fr compatibility.
The BIOTRONIK AMS-3 (drug-eluting AMS) system is comprised of a fast degradable carrier with a proven anti-proliferative drug. It is associated with increased developmental complexity due to interactions between the degradation kinetics of magnesium, the carrier, and the drug. The first animal trials with the AMS-3 prototype demonstrated safety, efficacy in the form of sustained anti-proliferative effect up to 90 days, and significant improvement in minimum lumen diameter at 14 and 28 days vs. bare AMS in the porcine model and similar late loss indices to clinically proven DES systems. The intent is to resume a first-in-man study with the AMS-3 in 2010 and to seek CE mark approval with the completion of the next round of clinical trials.
Summary
BIOTRONIK’s absorbable metal stent technology is based on a magnesium alloy that offers superior stent mechanics and biocompatibility. The first generation (AMS-1) showed promising results regarding mechanical properties as well as feasibility and safety in several human applications (150 cases). The second generation (AMS-2.1) shows improved scaffolding and efficacy in animals due to a more slowly degrading magnesium alloy and an optimised stent design. The preclinical results of the drug-eluting AMS-3 are encouraging and the clinical investigational program will resume in 2010.

