Introduction
In contrast to bare metal stents (BMS), polymer-based sirolimus-eluting stents (SES) and paclitaxel-eluting stents (PES) have both been shown to significantly reduce angiographic restenosis and recurrent ischaemia necessitating repeat revascularisation1. The occurrence of stent thrombosis, however, remains a clinical concern2,3. Furthermore, permanent metallic stenting may preclude surgical revascularisation, result in jailed side branches, prevent expansive remodelling, eliminate reactive vasomotion and impair the non-invasive imaging of coronary arteries with multislice computed tomography (MSCT) and magnetic resonance (MRI). As an alternative approach to the metallic drug-eluting stent, bioabsorbable polymer drug-eluting stents (DES) may provide short-term vessel scaffolding combined with drug delivery capability but avoid the long-term limitations of metallic stents.
The fully absorbable everolimus-eluting BVS stent (Abbott Vascular, Santa Clara, CA, USA) was tested in the first-in-man Absorb trial enrolling 30 patients with a single de novo coronary artery lesion. To assess the safety and vessel healing after implantation of the BVS stent, patients were clinically followed for two years using multi-modality imaging: MSCT, angiography, intravascular ultrasound (IVUS), IVUS radiofrequency backscattering and optical coherence tomography (OCT). In this article, we review the findings and insights from the Absorb (Cohort A) trial, and describe the currently ongoing Absorb Cohort B trial, and discuss future perspectives of this technology.
The BVS stent technology
The BVS stent design is characterised by a crossing profile of 1.4 mm with circumferential hoops of PLLA. The struts are 150 microns thick and are either directly joined or linked by straight bridges (Figure 1). Both ends of the stent have two adjacent radio-opaque platinum markers. The radial strength, measured in a water bath at 37°C using IVUS and by flat plate compression of 10, 15 and 25% is 0.048±0.007 N/mm2, 0.070±0.008 N/mm2 and 0.106±0.009 N/mm2, while comparative values for a contemporary bare-metal stent (Vision coronary stent, Abbott Vascular, Santa Clara, CA, USA) is 0.073±0.011 N/mm2, 0.114±0.012 N/mm2 and 0.155±0.012 N/mm2, respectively.
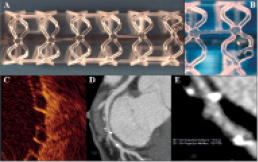
Figure 1. The BVS everolimus-eluting coronary stent system. (A) Overview of the stent. (B) Magnified image of the stent. (C) Stent struts imaged by optical coherence tomography have a “black box” appearance. The stent strut thickness is 150 µm. (D and E) Multi-slice computed tomography delineates easily the contours of the stented vessel as the stent structures are not radio-opaque. The radio-opaque markers embedded at the stent extremities (white arrows) appear much larger than they really are because of a “blooming effect” due to partial volume averaging typical of highly radio opaque objects imaged by MSCT4.
The backbone of BVS device is made of semicrystalline polymer called Poly-L-lactic acid4. The coating consists of poly D,L-lactide, which is a random copolymer of D- and L-lactic acid with lower crystallinity than the BVS backbone. The coating contains and controls the release of the anti-proliferative drug, everolimus. Both PLLA and PDLLA are fully bioabsorbable. During bioabsorption, the long chains of PLLA and PDLLA are progressively shortened as ester bonds between lactide repeat units are hydrolysed, and small particles less than 2 mm in diameter are phagocytosed by macrophages. Ultimately, PLLA and PDLLA degrade to lactic acid, which is metabolised via the Krebs cycle (Figure 2).
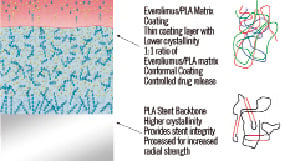
Figure 2. The backbone and coating of the BVS stents.
In a porcine coronary artery model,mass loss increased with time; 30% at 12 months increasing to 60% by 18 months post implantation.
Study design of the Absorb trial
The Absorb trial was a single-arm, prospective, open-label first-in-man study with safety and imaging endpoints. Between March and July 2006, 30 patients were enrolled at four participating sites. Inclusion criteria included (1) age ≥ 18 years; (2) a diagnosis of stable, unstable or silent ischaemia; (3) a single, de novo lesion in a native coronary artery of 3.0 mm, shorter than 8 mm for the 12 mm BVS or <14 mm for the 18 mm BVS (only two patients received the latter stent). Out of 30 patients enrolled, four patients were excluded from the per treatment evaluable population as they received a non-BVS stent. Clinical endpoints were assessed at six months, one and two years, while angiography, intravascular ultrasound (IVUS) and derived morphology parameters (virtual histology (VH), palpography and echogenicity) were assessed at six months and repeated at two years. Non-invasive coronary angiography with MSCT were performed at 18 months follow-up.
Is non-invasive evaluation of the treated vessel feasible?
In contrast to the radio-opaque metallic stents that hinder in-stent luminal assessment with MSCT by blooming artefacts, the polymeric BVS stent is radiolucent except for two metallic markers at both ends that allows for in-stent luminal assessment with MSCT along with an appreciation of the stented region (Figure 3). Reflecting the non-invasive nature of MSCT, 25 patients underwent MSCT imaging 18 months after the index procedure, which was more than the number of patients who underwent invasive coronary angiography (n=19). Out of the 25 patients who underwent MSCT, quantitative analysis was feasible in 24 patients. According to MSCT measurements, the mean luminal area was 5.2±1.3mm2, the minimal lumen area was 3.6±0.9 mm2 and the mean area stenosis was 34±15%. The calculated mean diameter stenosis was 19±9% and in fact it was not far from the results of invasive quantitative coronary angiography (% diameter stenosis, 27±11%). The feasibility and accuracy of the use of MSCT in analysing radiolucent biodegradable stents in a multicentre study may usher in a new era for non-invasive evaluation of patients treated with radiolucent stents5,6.
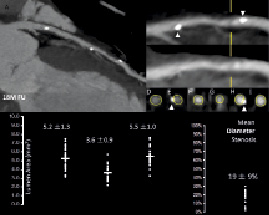
Figure 3. Example of MSCT images post-procedure, at 18 months after implantation (upper panel), and the results of quantitative analyses.
Does plaque deformability remain scaffolded at follow-up?
The underlying principle of palpography is that softer tissue is more readily deformed than harder or scaffolded tissue when pulsatile arterial pressure is applied7-10. The rationale of this analysis was to detect some subtle changes in strain resulting from scaffolding and late bioabsorption of the stent. The deformability of a vessel wall is quantified using back-scattering radiofrequency analysis of signals at different diastolic pressure levels. This allows for the reconstruction of a colour-coded ‘strain’ image in which harder (low strain in blue colour) and softer (high strain in yellow colour) regions of the coronary arteries can be identified, with radial strain values ranging between 0% and 2%.
At 2-year follow-up, the cumulative strain values showed no significant interval changes between six months and two years follow-up (0.28±0.12 vs. 0.31±0.17, p=0.80). These findings suggest that even at two years after implantation of BVS, plaque deformability remains scaffolded.
Does bioabsorption occur?
In the Absorb trial, multiple imaging modalities/analyses were used in an attempt to evaluate the bioabsorption of the polymeric struts: i) IVUS echogenicity ii) IVUS radio-frequency analysis (virtual histology) iii) optical coherent tomography11-13.
IVUS echogenicity
Based on IVUS echogenicity analyses, calcified plaques and polymeric stent struts were both recognised as hyperechogenic tissue. There was a significant reduction in percent hyperechogenicity between post-procedure and six months follow-up in ITT population (18.5±9.1% vs. 10.3±7.6%, p<0.001). Significant reduction was further observed between six months and 2-year follow-up (10.3±7.6% vs. 7.7±6.5%, p=0.005). More specifically, the level of degree of hyperechogenicity at two years was similar to that of natural plaques (5.6±4.8%, n=12) (Figure 4).
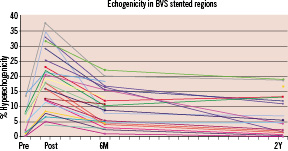
Figure 4. Changes in % hyperechogenic plaque up to two years according to echogenicity analysis. Courtesy of Dr. N. Bruining.
Virtual histology
IVUS backscattering radio-frequency analysis (virtual histology) was also applied to assess the absorption of the polymeric struts. Because polymeric struts are classified as dense calcium (DC) on VH immediately after implantation, it was hypothesised that bioresorption of struts would be reflected in a reduction of dense calcium at follow-up.
From pre- to post-stenting, there was an increase in the mean “DC” (9.8 vs. 25.4%, p<0.001) and “necrotic core (NC)” (15.5 vs. 30.5%, p=0.001) (n=13). At 6-month follow-up, VH showed a 30% decrease in “DC” (29.7% vs. 21.2%, p<0.001) and a nearly 20% decrease in “NC” (26.9 vs. 21.9%, p=0.005) (n=27)10. Between six months and 2-year follow-up, IVUS-VH assessments demonstrated no significant differences in percentage of each plaque component5.
Optical coherent tomography
Serial OCT data obtained immediately after stent implantation, at six months and 2-year follow-up were available in seven patients from the ITT population5. One of the main findings was a reduction in the number of apparent struts over time. The total number of apparent struts decreased from 403 at baseline to 368 at six months follow-up and to 264 at 2-year follow-up (35% reduction over two years). Stent strut appearance at two years is shown in Figure 5.
Although all imaging studies suggested (IVUS echogenicity, VH and OCT) a certain degree of bioabsorption, this would remain speculative without comparison of intracoronary images to histology. The correlation between changes in intracoronary images and histology need to be further investigated.
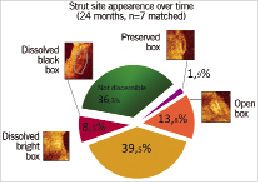
Figure 5. Appearance of the struts at two years after implantation.
Late luminal enlargement occurs
The main observation provided by grey-scale IVUS was the significant increase in minimal luminal area and average luminal area/volume together with a significant decrease in plaque area/volume between six months and 2-year follow-up. With the exception of the minimal luminal area (5.09 to 4.35 mm2, p=0.034), there were no apparent differences in the vessel area, average luminal area, plaque area as well as lumen area stenosis between the immediate post-procedure and 6-month follow-up measurements. Of note, the vessel area/volumes remained constant during follow-up suggesting the absence of significant remodelling. Late enlargement of the lumen was also observed in OCT analysis (n=7): Minimal and mean luminal area decreased significantly between post procedure and six months but enlarged significantly between six months and two years.
This observed phenomenon needs further investigation. The volumetric reduction in struts induced by bioresorption might explain this phenomenon. Otherwise, everolimus could exert a specific effect on the plaque to induce a reduction in plaque size between six months and 24-month follow-up by an autophagic effect14.
Restoration of vasomotion
To study vasomotion, either the endothelium independent vasoconstrictor methylergonovine maleate (methergine), or the endothelium dependent vasoactive agent, acetylcholine was administered at the time of 2-year angiographic follow-up. Mean lumen diameters were measured by QCA after baseline saline infusion and after administration of methergine/acetylcholine. Both tests were terminated by intracoronary administration of 200 micrograms of nitroglycerine.
In the methergine group (n=7), there was significant vasoconstriction in proximal (pre 2.70±0.43 mm vs. post Met 2.49±0.46 mm, p=0.02) and stented segments (pre 2.64±0.22 mm vs. post Met 2.44±0.33 mm, p=0.03). In the acetylcholine group (n=9), 5 patients exhibited vasodilation in the stented segment (Figure 6). These results suggested that there was restoration of the vasomotor function in the stented segment, an observation which has never been made after metallic stent implantation.
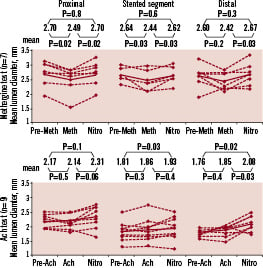
Figure 6. Changes in mean lumen diameter at methergine test (left panel) and at acetylcholine test (right panel). The middle graph in each panel shows mean luminal diameter in the stented segment.
The reappearance of vasomotion of the “stented” and persistent segments in response to methergine or acetylcholine indicates that vessel vasoreactivity has been restored and that a physiological response to vasoactive stimulus may occur anew. Furthermore, five out of nine patients tested with acetylcholine exhibited vasodilatation (at least 3 % of the mean diameter) during the highest infused doses, further suggesting direct vasodilator or flow-mediated response to acetylcholine and thus the presence of functionally active endothelium at the site of the stent implantation.
How about clinical outcomes?
At 2-years, clinical follow-up was obtained from 29 out of the 30 enrolled patients. At 2-year follow-up, there was only one non-Q wave myocardial infarction (peak troponin 2.21ng/ml) related to the treatment of a non-flow-limiting stenosis (QCA DS 42%) in a patient implanted with the BVS 46 days earlier. Furthermore, this patient experienced a single episode of angina at rest without any electrographic evidence of ischaemia. There were no new MACE events between six months and two years, and were no instances of stent thrombosis as defined by the protocol or ARC definitions15. In total, the MACE rate at two years was 3.4%.
Discovered challenges and future perspectives
During the Absorb trial, the mechanical properties of the polymeric stent were assessed. The acute recoil was evaluated in angiography as subtraction of the mean luminal diameter after balloon from the diameter at balloon inflation. The mean percent acute recoil was 6.85±6.96% for BVS in the Absorb trial, while it was 4.27±7.08% for Xience V stent (Abbott Vascular, Santa Clara, CA, USA) in the SPIRIT I trial16. This suggests that the mechanical properties to support the vessel wall are weaker in the BVS than in the Xience V stent.
The late recoil assessed by means of IVUS was defined as a reduction of the stent area from post procedure to 6-month follow-up (Figure 7)17. At six months, the lumen area was reduced by 16.6%, while the late recoil was 11.7%. This suggested that approximately two-thirds of the luminal area reduction was caused by late recoil.
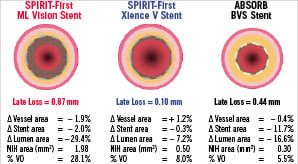
Figure 7. Changes from post-procedure to 6-month in vessel area, stent area, lumen area, neointimal hyperplasia (NIH) area and % volume obstruction (VO) in multilink vision bare-metal stent (left panel), in Xience-V everolimus-eluting stent (middle panel) and in BVS stent (right panel).
Modification of strut design
To enhance the mechanical properties of the struts and to reduce either acute or late recoil, design iterations were made to the struts of the BVS stent platform (revision 1.1) as indicated in Figure 8. The BVS revision 1.1 uses the same polymers in both the scaffold and coating as the original 1.0 design, and has the same radiolucent platinum markers. The polymer scaffold is able to provide radial support for longer periods, while retaining the same total time required for complete absorption at two years. The strut thickness remains the same; however, the new design has in-phase zigzag hoops linked by bridges. These design changes allow for a more uniform strut distribution, which reduces maximum circular unsupported surface area (MCUSA) and provides greater/more uniform vessel wall support and drug transfer. The BVS revision 1.1 is being used in the ABSORB Cohort B study which is a multicentre, non-randomised trial to assessment of the safety and performance of the BVS revision 1.1, in the treatment of patients with a maximum of two de novo native coronary artery lesions (3.0 mm in diameter and ≤ 14 mm in length). The ABSORB Cohort B will enrol approximately 80 patients in approximately 10 sites in the European Union and Asia Pacific region. These 80 patients will be divided into two groups, the 1st group (~ 40 patients) having imaging follow-up procedures performed at 180 days and two years and the 2nd group (~ 40 patients) having imaging follow-up procedures performed at one year and two years. Enrolment started on March 19, 2009, and is expected to end in November 2009.
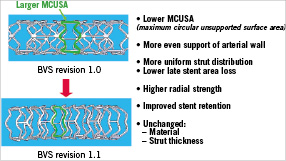
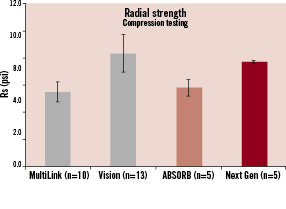
Figure 8. The top panel indicates the modification of stent design from revision 1.0 to revision 1.1. In the revision 1.1, the maximum circular unsupported stent area was reduced to reduce loss in the stent area. As a result, revision 1.1 has a higher radial strength than revision 1.0 (bottom panel) and similar radial strength with the Vision metallic stent.
Conclusion
In summary, the Absorb trial showed the following phenomena: 1) stented lesion can be assessed by non-invasive imaging (MSCT); 2) bioabsorption does occur (echogenicity, VH & OCT); 3) late enlargement of lumen has been documented (IVUS & OCT); 4) plaque deformability remains scaffolded at follow-up (high-strain spot on palpography); 5) vasomotion and endothelial function can be restored (angiography + ach/ methergine); 6) 2-year MACE rate was as low as 3.4% without any stent thrombosis. To further enhance the mechanical properties of the polymeric struts and to reduce recoil, the strut design was modified and resulted in the new BVS revision 1.1. This latest revision of the BVS stent is being tested in the ABSORB cohort B trial, whose enrolment is expected to finish in November 2009.

