
Abstract
Aims: The one-year randomised data of the ABSORB II trial showed that the everolimus-eluting bioresorbable scaffold and the everolimus-eluting metallic stent were comparable for the composite secondary clinical outcomes of patient-oriented composite endpoint (PoCE) and device-oriented composite endpoint (DoCE)/target lesion failure (TLF), MACE and TVF. This report describes the two-year clinical outcomes of the ABSORB II trial.
Methods and results: Patients were randomly assigned in a 2:1 ratio to receive treatment with an everolimus-eluting bioresorbable scaffold (Absorb; Abbott Vascular, Santa Clara, CA, USA) or treatment with an everolimus-eluting metallic stent (XIENCE; Abbott Vascular). The trial enrolled 501 patients. Clinical follow-up at two years was available in 320 patients in the Absorb BVS arm and 160 patients in the XIENCE arm. At two years, the PoCE for the Absorb and XIENCE arms was 11.6% and 12.8% (p=0.70) and the DoCE/TLF was 7.0% and 3.0% (p=0.07), respectively. The hierarchical ID-MACE rate was 7.6% vs. 4.3% (p=0.16) and the rate of TVF was 8.5% vs. 6.7% (p=0.48). The definite/probable thrombosis rate was 1.5% in the Absorb arm vs. 0% in the XIENCE arm (p=0.17). Thirty-six percent and 34% of patients remained on DAPT at two years, respectively. Ninety-two percent of patients in both arms remained on aspirin.
Conclusions: Two-year clinical results demonstrate sustained low rates of PoCE, MACE, DoCE and TVF with the Absorb BVS as compared to the XIENCE stent.
Introduction
The Absorb™ bioresorbable polymeric everolimus-eluting scaffold (Absorb BVS; Abbott Vascular, Santa Clara, CA, USA) was developed as an alternative to metallic drug-eluting stents with the aim of providing transient vessel support combined with drug delivery capability.
The first-generation Absorb everolimus-eluting bioresorbable vascular scaffold was tested in the first-in-man ABSORB Cohort A trial which demonstrated low clinical event rates up to five-year follow-up1. Following this, improvements were introduced to the design in order to improve angiographic late loss at 180 days and manufacturability, without differences in polymeric material, drug dose, drug release or strut thickness. The performance of this next-generation Absorb BVS was investigated in the ABSORB Cohort B trial which reported excellent clinical results up to five-year follow-up2.
The ABSORB II randomised controlled trial (ClinicalTrials.gov NCT01425281; study sponsor Abbott Vascular) was initiated to compare the Absorb BVS with the metallic everolimus-eluting XIENCE stent (Abbott Vascular).
Methods
STUDY DESIGN
The study design and device specifications have been reported in detail previously3. Briefly, in this prospective, randomised, active controlled, single-blinded, multicentre trial 501 patients were randomised in a 2:1 fashion to the Absorb BVS or the XIENCE stent (335 and 166 patients in each arm, respectively). The trial protocol allowed the treatment of up to two de novo native coronary artery lesions, each to be located in different major epicardial vessels, with a maximal lumen diameter between 2.25 mm and 3.8 mm as assessed by online QCA and a maximum lesion length of ≤48 mm. All subjects were screened as per the protocol inclusion and exclusion criteria and were required to provide signed informed consent prior to enrolment. All subjects are to have clinical follow-up at 30 and 180 days and at 1, 2, 3, 4 and 5 years and are to undergo coronary angiography, intravascular ultrasound (IVUS), and IVUS virtual histology (VH) imaging pre and post device implantation and at three years post index procedure. All major adverse cardiac events (MACE) will be adjudicated by an independent clinical events committee.
STUDY DEVICES
The XIENCE stent and Absorb BVS (both Abbott Vascular) share the same MULTI-LINK design, and both devices are similar in terms of drug, drug dose density, and elution profile3.
STATISTICAL ANALYSIS
The sample size and power calculation for this study have been reported previously3. For binary variables, counts and percentages were calculated. For exact 95% CIs we used the Clopper-Pearson method. Continuous variables are summarised with means and SDs. For 95% CIs for the mean, we used the Gaussian approximation. The chi-square test or Fisher’s exact test was used to compare binary variables, and the Student’s t-test or non-parametric test was used to compare continuous variables. All p-values in this two-year report are two-tailed and are for descriptive purposes; no formal hypothesis testing was done. The analysis presented in this report is based on the intention-to-treat population. All statistical analyses were performed using SAS software, version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Patient demographics and a flow chart are shown in Table 1 and Figure 1, respectively. Four hundred and eighty patients (95.8%) underwent clinical follow-up at two years. Clinical outcomes up to one-year follow-up have been described elsewhere4. Hierarchical composite clinical event rates up to two years are presented in Table 2.
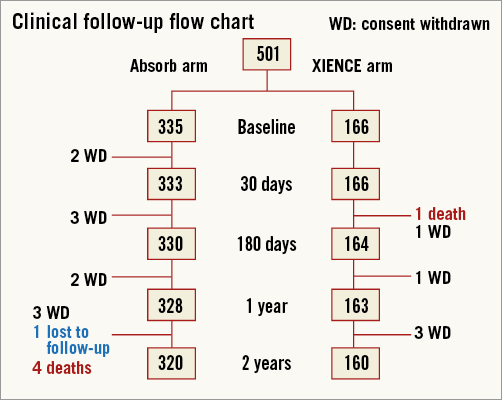
Figure 1. Flow chart of patient follow-up.
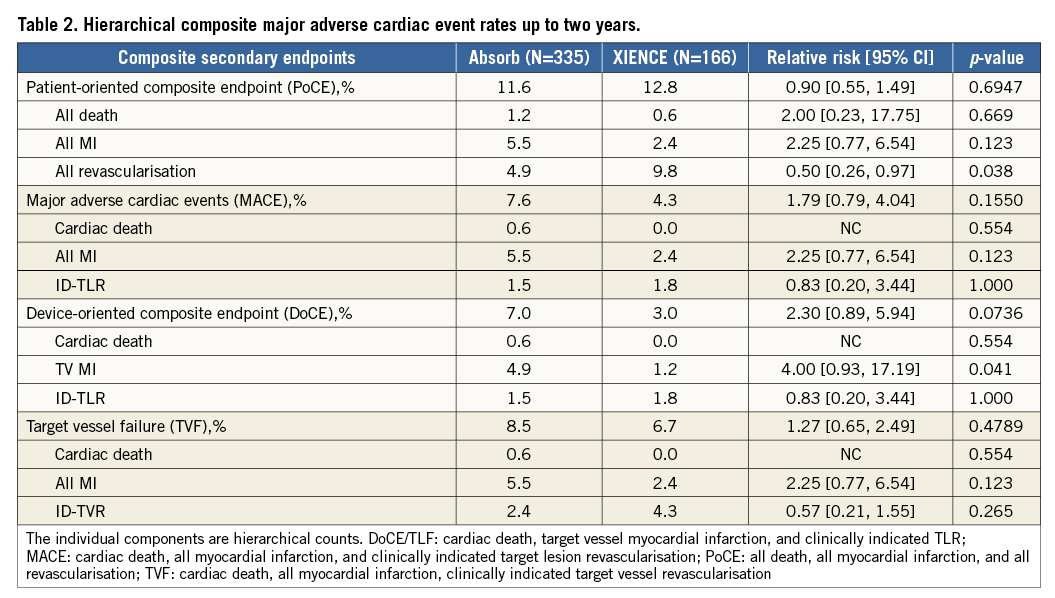
There were no statistically significant differences in the composite MACE rate between the two arms: 7.6% in the Absorb BVS arm and 4.3% in the XIENCE arm (p=0.16). Also, there were no statistically significant differences in the device-oriented composite endpoint (DoCE) or target lesion failure (TLF) between the Absorb BVS and XIENCE arms: 7.0% and 3.0%, respectively (p=0.07). Finally, the patient-oriented composite endpoint (PoCE), or DMR (death, MI and revascularisation), was similar in both arms: 11.6% in the Absorb BVS arm and 12.8% in the XIENCE arm (p=0.69).
A landmark analysis of time-to-event curves at 37 days showing the cumulative incidence of DoCE, MACE, PoCE and TVF is shown in Figure 2.
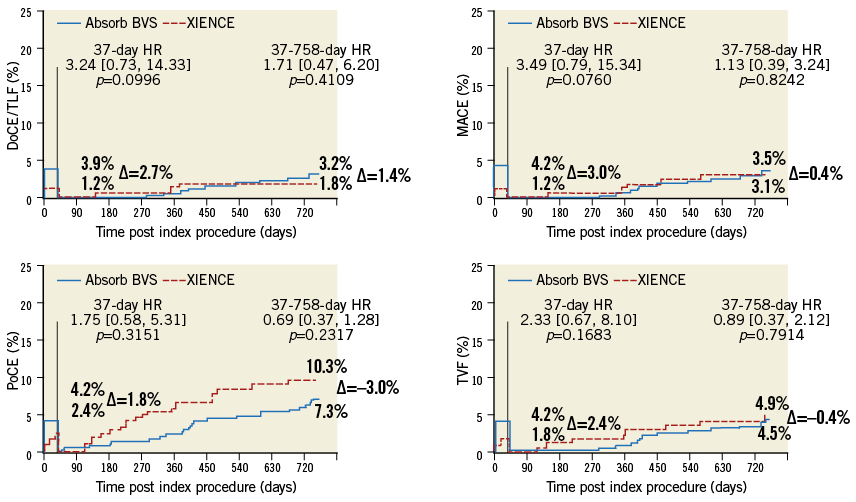
Figure 2. Time-to-event curves of the composite secondary endpoints.
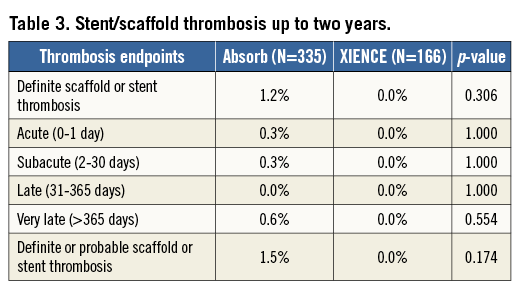
Stent/scaffold thrombosis rates up to two years are presented in Table 3. There was no statistically significant difference between the two arms. In the Absorb arm, up to two years, there was one definite acute and one definite subacute scaffold thrombosis, one probable late scaffold thrombosis and two definite very late scaffold thromboses. There were no stent thrombosis events in the XIENCE arm.
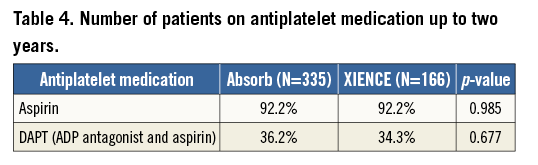
The number of patients on antiplatelet medication up to two years is presented in Table 4. At two years, 36% of the patients in the Absorb BVS arm were on dual antiplatelet therapy (DAPT), while 34% of the XIENCE arm remained on DAPT.
Discussion
This two-year interim analysis of the clinical results of the ABSORB II study shows that there is no significant difference between the Absorb BVS and the XIENCE stent in terms of the rates of composite clinical endpoints (PoCE, DoCE, MACE and TVR). At two years, the rates of death, myocardial infarction and device thrombosis did not vary significantly between the two devices, although the rate of scaffold thrombosis was higher after implantation of the Absorb scaffold.
In the current study, procedural and device success rates were similar between the two arms (96% vs. 99% and 99% vs. 100% in the Absorb and XIENCE arms, respectively)4. However, there was a difference in the rate of myocardial infarction (4% vs. 1%, respectively), primarily driven by a cardiac biomarker rise within 48 hours of the procedure. In order to eliminate that effect on the two-year results the landmark analysis was performed.
The low rates of the device-oriented composite clinical endpoint of TLF at one year (BVS 5% vs. XIENCE 3%)4 were maintained up to two years (BVS 7% vs. XIENCE 3%). Similarly, the PoCE at one year (BVS 7% vs. XIENCE 9%)4 was maintained up to two years (BVS 11.6% vs. XIENCE 12.8%). The one-year rate of TLF is comparable to that of other ABSORB randomised controlled trials (RCT), namely ABSORB III (BVS 7.8% vs. XIENCE 6.1%)5, ABSORB China (BVS 3.4% vs. XIENCE 4.2%)6 and ABSORB Japan (BVS 4.2% vs. XIENCE 3.8%)7. There are no long-term RCT data available in the literature to compare the two-year results of the ABSORB II study. Despite a non-significant numerical difference in TLF rates in favour of the XIENCE stent, it is important to highlight that these studies are comparing the second generation only of BVS with the best in class metallic stent XIENCE. There was a significant difference noted in the rate of all revascularisations where the rate was higher in the XIENCE arm (9.8%) compared to the BVS arm (4.9%). This was due to higher rates of non-target vessel revascularisation noted in the XIENCE arm (6.7%) compared to the BVS arm (3.0%). There were no statistically significant differences in the rates of TLR and TVR.
The rate of scaffold thrombosis was higher in the Absorb arm but this was not statistically significant. The rate of early ST (acute and subacute ST) in this study (BVS 0.6% vs. XIENCE 0.0%) is comparable to that seen in other RCT, namely ABSORB III (BVS 1.1% vs. XIENCE 0.7%)5, ABSORB China (BVS 0.4% vs. XIENCE 0.0%)6 and ABSORB Japan (BVS 1.1% vs. XIENCE 0.8%)7. In the meta-analysis of the four randomised trials, the definite or probable device thrombosis rates up to one year were 1.3% and 0.6% in the Absorb and the XIENCE arms, respectively8. The early scaffold thrombosis is potentially related to microcirculation disturbance due to protruding or malapposed struts of the Absorb scaffold. Because of its relatively thick strut, dedicated implantation techniques are indispensable to optimise the acute and midterm results9.
There are limited or no published data on definite very late ST from any RCT. There were two cases of very late scaffold thrombosis in the Absorb arm. The first case was a 44-year-old male with a history of dyslipidaemia, hypertension, and MI, and he was a former tobacco user. A 13.6 mm lesion in the proximal LAD (Dmax=3.01 mm) was predilated and one BVS (3.0×18 mm BVS at 10 atm) was implanted, followed by post-dilatation (3.25×10 mm at 10 atm). On post-procedural IVUS, strut malapposition was observed in the proximal part of the scaffold, which remained uncorrected. The angiographic residual stenosis was 21%, suggesting the suboptimal expansion of the scaffold. On day 447 the patient presented to the emergency room with chest pain and a ventricular tachycardia. ECG revealed ST-elevation in the anterolateral leads and a catheterisation was performed which revealed thrombotic occlusion of the ostial and proximal LAD. Thrombo-aspiration was performed followed by DES implantation. The patient was on aspirin only at the time of the event. In the second case, a 54-year-old male with a history of dyslipidaemia, hypertension and former tobacco use was treated. Two lesions, one 14 mm lesion in the mid LAD (Dmax=2.7 mm) and one 14 mm lesion in the mid RCA (Dmax=3.0 mm), were predilated and one BVS (3.0×18 mm at 10 atm) was implanted in the mid LAD and one BVS (3.0×18 mm at 7 atm) in the mid RCA. Post-dilatation was not performed in either of the lesions. On post-procedural IVUS, the proximal edge of the scaffold was landed in a diseased segment (plaque burden of 69.8%), indicating that the proximal plaque remained uncovered by scaffold. In addition, the residual area stenosis on IVUS was 31.8%, suggesting that the expansion of the device was suboptimal. On day 599 the patient presented to the emergency room with chest pain, and ST changes in the inferior leads. Angiography was performed on day 602 which revealed a patent scaffold in the mid LAD and in-scaffold occlusion in the mid RCA. The core lab assessed a potential stent thrombus in the distal RCA with 100% stenosis. Thrombo-aspiration was performed and a DES was implanted in the mid RCA. Aspirin was ongoing at the time of the event while clopidogrel was stopped after one year.
The mechanism of very late scaffold thrombosis (VLST) still remains to be clarified. The previous case series with intravascular imaging suggested that factors such as incomplete lesion coverage, malapposition, strut discontinuity and underexpansion seem to play an important role, although the frequency and clinical impact of such imaging findings still need to be determined10. In a case series of 14 patients presenting with definite BVS thrombosis, the role of dual antiplatelet medication was also highlighted in five of the 14 cases10. The complete bioresorption of the scaffold takes three to four years, which could be associated with sustained inflammation in histology11. In addition, these two-year results of the ABSORB II study underline the importance of correct implantation technique, such as, for example, correct sizing and high-pressure post-dilatation, which are important factors that may not have been fully appreciated at the time of enrolment into the ABSORB II study. Long-term data from large RCT might provide insights into the incidence of VLST events.
Limitations
The generalisability of the study to daily practice is limited due to the selected population included in the study. The study is also severely underpowered to detect a difference in any clinical events, especially low-frequency events such as scaffold/stent thrombosis. This two-year interim analysis of the clinical results of the ABSORB II study was not a pre-specified endpoint. Additionally, investigators’ longer experience with the XIENCE stent as compared to Absorb BVS may have impacted on the results.
Conclusion
The two-year clinical results demonstrate sustained low rates of PoCE, MACE, DoCE and TVF with the Absorb BVS compared to the XIENCE stent. Long-term data from large RCT might provide insights into the incidence of VLST events.
| Impact on daily practice The meta-analyses of one-year results of randomised trials comparing a bioresorbable scaffold and a metallic stent consistently showed comparable clinical outcomes between the two devices at one year; however, there were scarce data about midterm results after one year. In the randomised ABSORB II trial comparing a bioresorbable scaffold and a metallic stent, the two-year clinical results did not differ between the two arms and demonstrated sustained low rates of clinical events, such as patient-oriented composite endpoints, in both arms. The rare occurrence of very late scaffold thrombosis events warrants further investigation. |
Guest Editor
This paper has been guest edited by Tommaso Gori, MD, PhD; Zentrum für Kardiologie, Universitätsmedizin Mainz, University Medical Center, Mainz and DZHK Rhein-Main, Germany.
Conflict of interest statement
B. Chevalier is a consultant for Abbott Vascular. A. van Boven has received research support and speaker fees from Abbott Vascular. J. Piek is a member of the Abbott Vascular Advisory Board. Y. Onuma and P. Serruys are members of the Abbott Vascular Advisory Board. R. Kumar is an employee of Abbott Vascular. L. Wasungu is a contractor from Novellas Healthcare working for Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor, Tommaso Gori, has received speaker’s honoraria from multiple companies including Abbott Vascular.

