
Abstract
Aims: The aim of this report of the AIDA trial is to provide full two-year outcomes for the primary endpoint of target vessel failure (TVF) and an update on device thrombosis.
Methods and results: AIDA was a single-blind, multicentre, investigator-initiated, non-inferiority, randomised (1:1) clinical trial. At complete two-year follow-up, the primary endpoint of TVF had occurred in 100 patients in the Absorb BVS arm versus 90 patients in the XIENCE EES arm (HR 1.12, 95% CI: 0.94-1.49; psuperiority=0.436). Estimated two-year Kaplan-Meier event rates of TVF were 11.0% and 9.9%, respectively (95% CI: −0.9%-3.0%; pnon-inferiority=0.003). Definite or probable device thrombosis at two years occurred in 30 patients in the Absorb BVS arm and in eight patients in the XIENCE EES arm. Kaplan-Meier estimates of device thrombosis were 3.3% in the Absorb BVS arm and 0.9% in the XIENCE EES arm (HR 5.22, 95% CI: 2.00-13.59; p<0.001).
Conclusions: AIDA formally met its criterion for non-inferiority of Absorb BVS versus XIENCE EES in terms of the combined endpoint of TVF. The Absorb BVS, however, was associated with higher rates of scaffold thrombosis and target vessel myocardial infarction at complete two-year follow-up.
Abbreviations
BVS: bioresorbable vascular scaffold
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
DSMB: data and safety monitoring board
EES: everolimus-eluting stent
PCI: percutaneous coronary intervention
ScT: scaffold thrombosis
TLF: target lesion failure
TVF: target vessel failure
TVMI: target vessel myocardial infarction
Introduction
Coronary bioresorbable vascular scaffolds were developed in order to overcome the shortcomings of conventional coronary metallic drug-eluting stents (DES)1. The most widely used and studied bioresorbable vascular scaffold was the Absorb everolimus-eluting bioresorbable vascular scaffold (Absorb BVS; Abbott Vascular, Santa Clara, CA, USA). The Absorb BVS received the Conformité Européenne (CE) mark in 2010 and was approved by the Food and Drug Administration (FDA) in 2016. While it gained acceptance in ordinary practice, no adequately powered, randomised, all-comers study addressing the safety and efficacy of the Absorb BVS had been performed at the time of commercialisation. Between 2013 and 2015 we performed the Amsterdam Investigator-initiateD Absorb strategy (AIDA) trial comparing the Absorb BVS with the XIENCE everolimus-eluting stent family (XIENCE EES; Abbott Vascular) in a population reflecting daily clinical practice with a primary endpoint of target vessel failure (TVF) at two years.
The data and safety monitoring board (DSMB) observed an increased rate of early and late scaffold thrombosis (ScT) and recommended considering prolonged dual antiplatelet therapy (DAPT) in all patients treated with Absorb BVS, as well as early reporting of the then available outcomes. This recommendation was implemented and the preliminary results with a median follow-up of 707 days were published2. In this manuscript, we report the full two-year primary outcomes and all clinical outcomes following treatment with Absorb BVS and XIENCE EES in the AIDA trial.
Methods
STUDY DESIGN
The AIDA trial was a single-blind, multicentre, investigator-initiated, non-inferiority, randomised (1:1) clinical trial. The study design and oversight of the AIDA trial have been described previously3.
AIDA enrolled patients with coronary artery disease who were eligible for inclusion if they were undergoing PCI, and were suitable candidates for treatment with a DES in accordance with the applicable guidelines and instructions for use (IFU) of the Absorb BVS and XIENCE EES families. Key exclusion criteria were target lesions longer than 70 mm, a reference vessel diameter of <2.5 mm or >4.0 mm estimated visually and treatment of bifurcation lesions in which a two-device strategy was planned, and treatment of in-stent restenosis. All included patients provided oral and written informed consent. In case of urgent PCI, oral consent was given before randomisation, and full written consent was obtained after the procedure.
After successful predilatation of the first lesion, patients were randomised to either Absorb BVS or XIENCE EES. Randomisation was performed with the use of a centralised web-based system in random block sizes. Patients were blinded to the study-group assignment, operators were not. During the first year of enrolment, scaffolds were implanted according to the manufacturer’s IFU, which, at that time, did not include mandatory post-dilatation. Post-dilatation was performed in 63% of the lesions in the scaffold group during the first year of enrolment. After the IFU changed, the steering committee recommended routine post-dilatation of the Absorb BVS-treated lesions from October 2014 onwards. DAPT was administered according to the guidelines of the European Society of Cardiology and the IFU of the Absorb BVS or the XIENCE EES. DAPT was recommended for at least one year after scaffold implantation in patients with acute coronary syndrome. Due to unexpected higher rates of ScT, the DSMB recommended a cross-sectional data sweep in November 2016 and consequent publication of the descriptive information (without formal hypothesis testing) on all endpoint events that occurred before December 2016 2. At the time of the publication of the preliminary results, all patients were unblinded and informed.
Clinical endpoints have been described previously and included the primary endpoint of target vessel failure (TVF), a composite of cardiac death, myocardial infarction and target vessel revascularisation, at two years (powered for non-inferiority)3. Secondary endpoints were all-cause death, all myocardial infarctions, all revascularisations, and device thrombosis. All myocardial infarctions were defined by the third universal definition of myocardial infarction and all other events were defined according to the Academic Research Consortium definitions4,5. An independent clinical events committee adjudicated all reported adverse events. Follow-up is ongoing annually up to five years, and is currently complete up to two years. Baseline SYNTAX score calculations were performed by the core laboratory staff of Cardialysis BV (Rotterdam, the Netherlands).
STATISTICAL ANALYSIS
This report provides the pre-specified two-year primary endpoint analysis from the AIDA trial. The AIDA trial was designed to test whether Absorb BVS was non-inferior to XIENCE EES, as determined by the rates of TVF at two years. To satisfy the non-inferiority hypothesis, the upper limit of the (two-sided) 95% confidence interval for the rate difference (equivalent to non-inferiority testing at a one-sided alpha level of 2.5%) had to fall below a pre-specified margin of 4.5 percentage points. Under the assumption of a 7.3% event rate for the primary endpoint at two years and a rate of loss to follow-up of 3.0%, we estimated that we would need to enrol 1,790 patients for the study to have at least 95% power. The first version of the protocol included a non-inferiority margin of 3.3 percentage points, which required enrolment of 2,690 patients for 90% power. After publication of the results of the ABSORB III trial, we amended the protocol, on the basis of FDA guidance, to adopt the non-inferiority margin of 4.5 percentage points used in that trial6. At the time that the protocol change was approved by the institutional review board in December 2015, a total of 1,845 patients had been enrolled, and enrolment was complete.
Analyses of primary and secondary endpoints were performed according to the intention-to-treat principle. Two-year event rates were based on Kaplan-Meier estimates in time-to-event analyses. Follow-up of the patients was censored at 730 days or at the last known event-free time point, whichever came first. For time-to-event analysis, hazard ratios with 95% confidence intervals were determined, and Kaplan-Meier curves were compared by means of the log-rank test.
For non-inferiority testing of the primary endpoint of TLF, we calculated the 95% confidence interval for the rate difference (the rate in the scaffold group minus the rate in the stent group) according to the method of Com-Nougue with the use of two-year Kaplan-Meier estimates of the TVF rate and Greenwood estimators of the standard error. We used the chi-square test or Fisher’s exact test to compare categorical variables and the independent t-test to compare continuous variables. All statistical analyses were performed with SPSS software, Version 23.0 (IBM Corp., Armonk, NY, USA).
Results
Between August 2013 and December 2015, AIDA enrolled 1,845 patients, of whom 924 were randomised to treatment with Absorb BVS and 921 to XIENCE EES. The baseline characteristics in the two study arms were well balanced and have been reported previously2 (Table 1). At complete two-year follow-up, clinical status was known in 96.9% of the patients (Supplementary Figure 1).
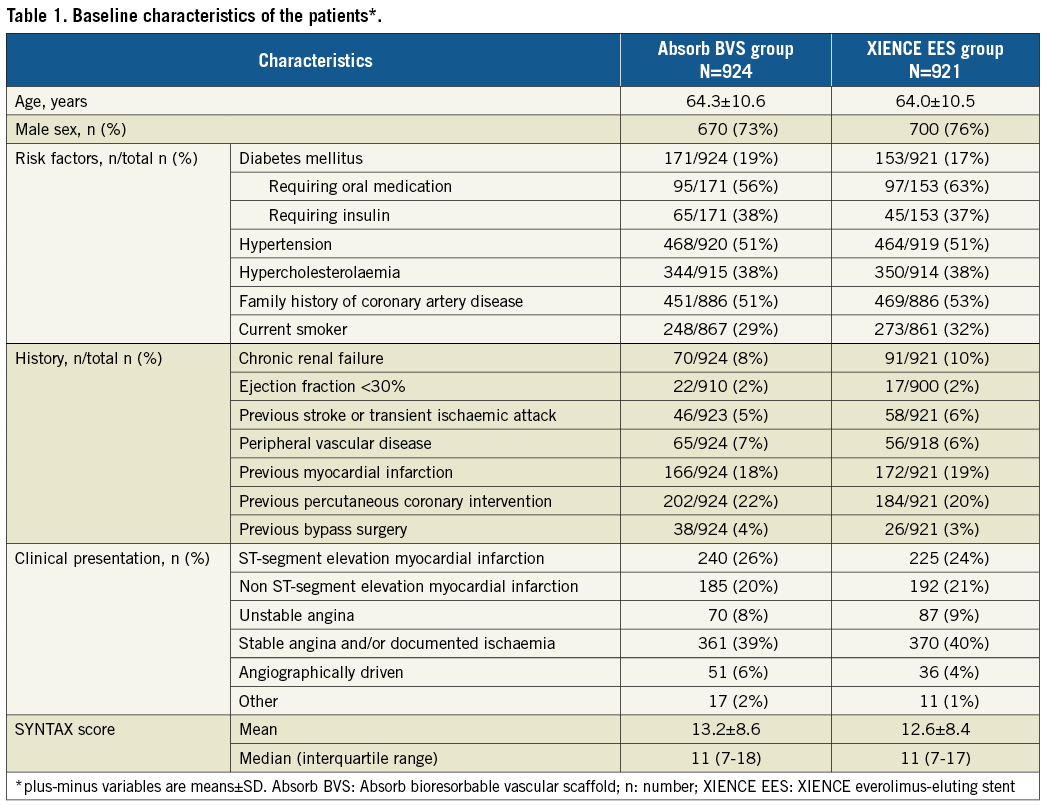
Procedural characteristics have been reported previously2 (Table 2). Briefly, a total of 2,446 lesions were treated. Successful implantation of at least one or more study devices was achieved in 895/924 (96.7%) patients in the Absorb BVS arm and in 919/921 (99.8%) patients in the XIENCE EES arm. Only assigned study devices were implanted in 859/924 (93.0%) patients in the Absorb BVS arm versus 910/921 (98.8%) in the XIENCE EES arm. The characteristics of the treated lesions are listed in Supplementary Table 1.
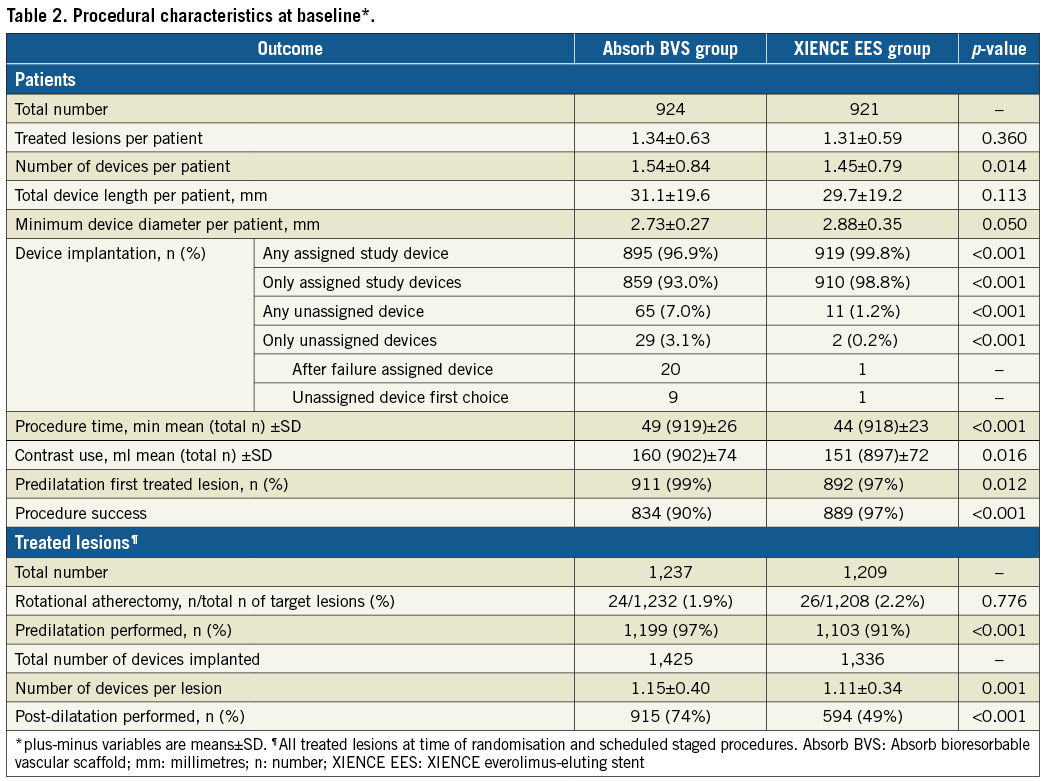
At complete two-year follow-up, TVF had occurred in 100 patients in the Absorb BVS arm versus 90 patients in the XIENCE EES arm (HR 1.12, 95% CI: 0.94-1.49; psuperiority=0.436). Estimated two-year Kaplan-Meier event rates of TVF were 11.0% and 9.9%, respectively, 95% CI: –0.9%-3.0%; pnon-inferiority=0.003) (Figure 1, Table 3). Cardiac death occurred in 17 patients in the Absorb BVS arm and in 20 patients in the XIENCE EES arm, 1.9% and 2.2%, respectively (HR 0.85, 95% CI: 0.44-1.62; p=0.618). Rates of target vessel myocardial infarction (TVMI) were 5.1% in the Absorb BVS arm and 3.1% in the XIENCE EES arm (HR 1.65, 95% CI: 1.03-2.64; p=0.034). Rates of target vessel revascularisation (TVR) were 8.2% in the Absorb BVS arm and 7.0% in the XIENCE EES arm (HR 1.18, 95% CI: 0.84-1.65; p=0.333). Rates of TLR were 6.5% in the Absorb BVS arm and 4.8% in the XIENCE EES arm (HR 1.35, 95% CI: 0.91-1.99; p=0.133). Rates of TLR caused by device thrombosis were 2.8% in the Absorb BVS arm and 0.5% in the XIENCE EES arm (HR 5.02, 95% CI: 1.92-13.10; p<0.001). Rates of TLR caused by device stenosis were 3.9% in the Absorb BVS arm versus 4.3% in the XIENCE EES arm (HR 0.89, 95% CI: 0.57-1.41; p=0.626).
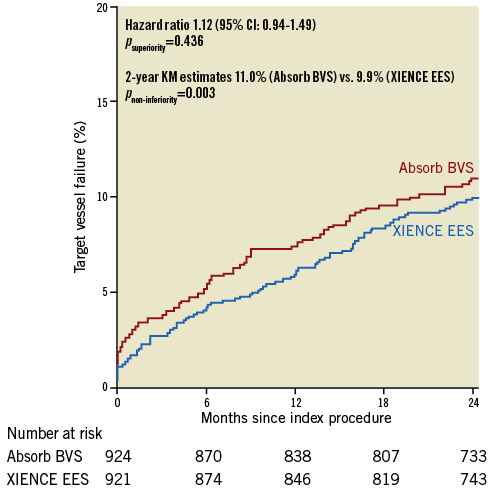
Figure 1. Kaplan-Meier curve for the composite endpoint of target vessel failure. Absorb BVS: Absorb bioresorbable vascular scaffold;
KM: Kaplan-Meier; XIENCE EES: XIENCE everolimus-eluting stent
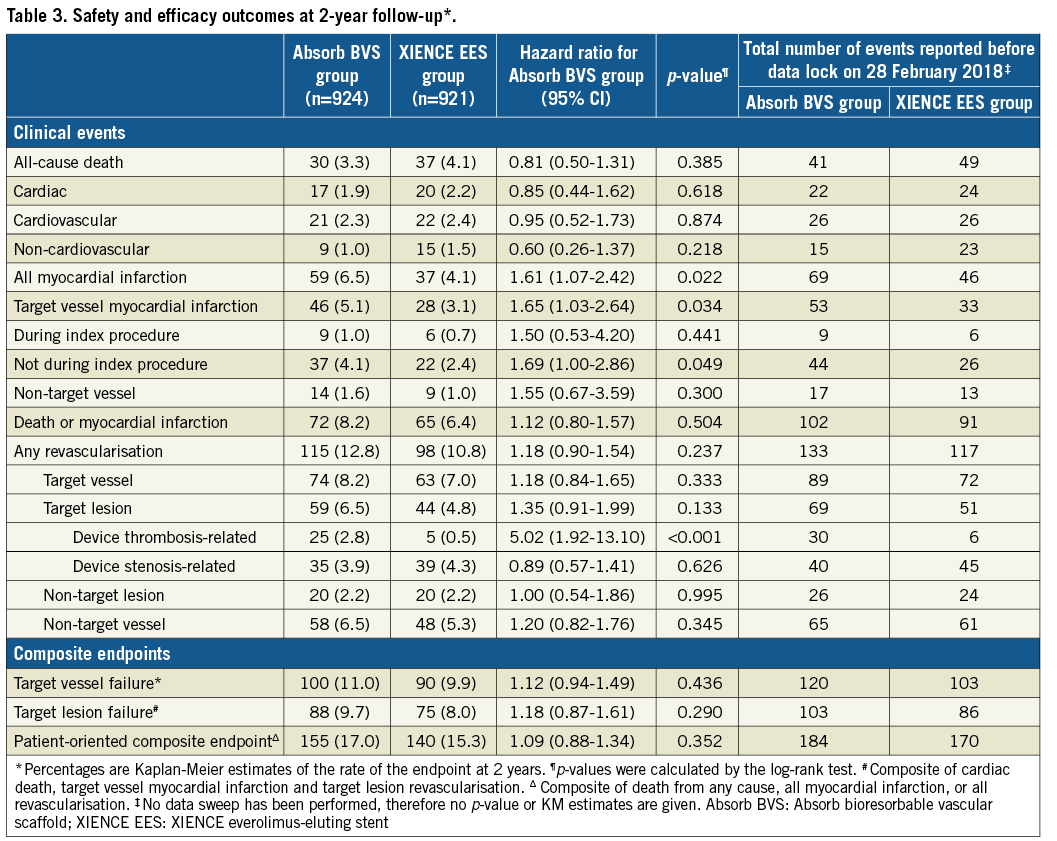
Definite or probable device thrombosis at two years occurred in 30 patients in the Absorb BVS arm and in eight patients in the XIENCE EES arm. Kaplan-Meier estimates of device thrombosis were 3.3% in the Absorb BVS arm and 0.9% in the XIENCE EES arm (HR 5.22, 95% CI: 2.00-13.59; p<0.001) (Table 4, Figure 2, Supplementary Table 2). At median follow-up of 1,092 days, definite or probable device thrombosis occurred in 35 patients in the Absorb BVS arm versus nine patients in the XIENCE EES arm. Very late device thrombosis occurred in 14 patients in the Absorb BVS arm versus three patients in the XIENCE EES arm. The 30-day landmark analysis for definite or definite and probable device thrombosis is shown in Supplementary Figure 2. The subgroup analysis of definite and probable ScT is shown in Supplementary Figure 3. Event rates at one year, and between one and two years, are shown in Supplementary Table 3 and Supplementary Table 4, respectively. Event rates of the “as treated population” and the “per protocol treatment population” are shown in Supplementary Table 5 and Supplementary Table 6, respectively.
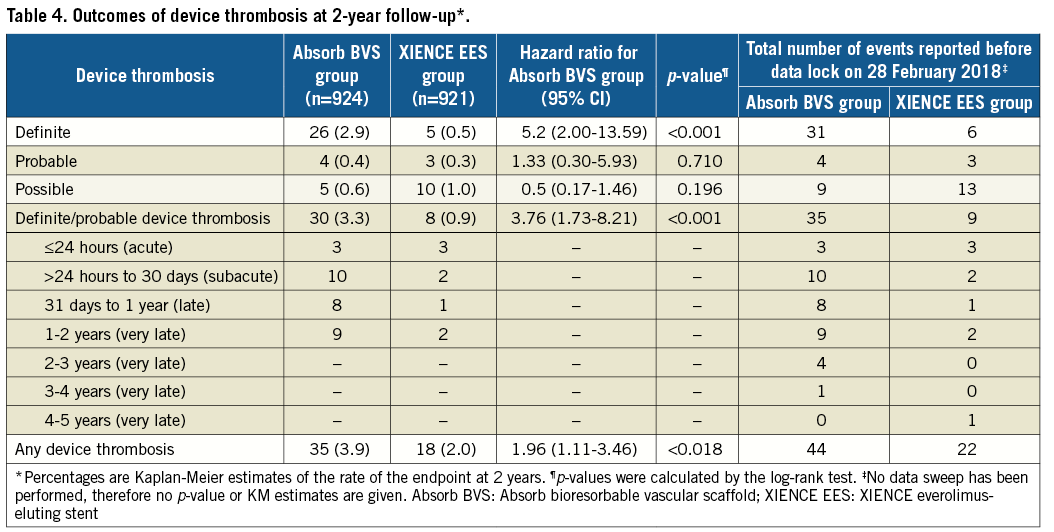
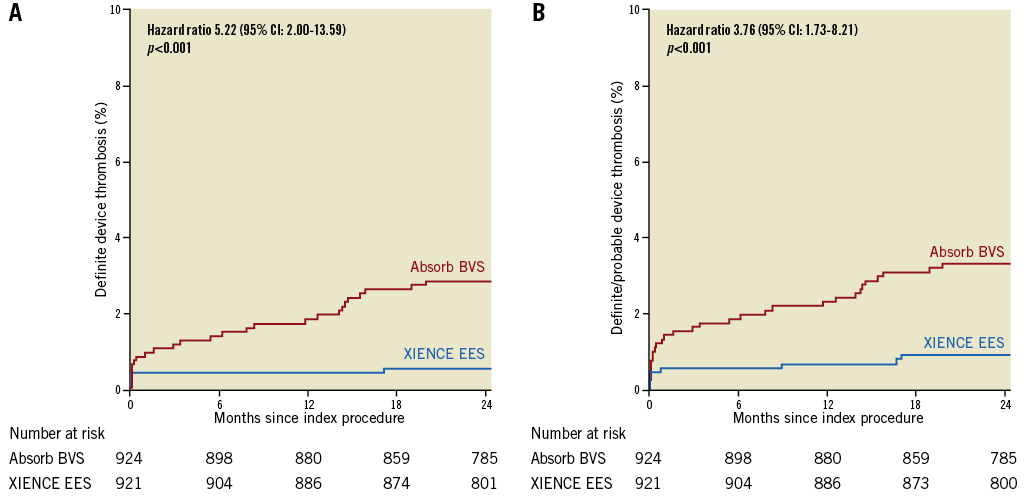
Figure 2. Kaplan-Meier curve analysis. A) Definite device thrombosis. B) Definite or probable device thrombosis. Absorb BVS: Absorb bioresorbable vascular scaffold; XIENCE EES: XIENCE everolimus-eluting stent
Discussion
First, we found a slightly increased rate of TVF at two years (11.0% versus 9.9%) in Absorb BVS. Nevertheless, the 95% CI for the rate difference in TVF fell below the pre-specified non-inferiority criterion of 4.5%. So, AIDA formally met its criterion for non-inferiority of Absorb BVS versus XIENCE EES in terms of TVF. Second, we found that Absorb BVS was associated with higher two-year rates of ScT and related myocardial infarction. Third, rates of TLR due to device thrombosis were statistically significantly higher in the Absorb BVS arm (2.8% vs. 0.5%; HR 5.02, 95% CI: 1.92-13.10; p<0.001). The rates of TLR not due to device thrombosis were similar.
The initial results of the first registries and clinical randomised trials with the Absorb BVS showed promising results with acceptable rates of TLF and ScT at one year. Initial enthusiasm was dampened when various studies reported increased rates of ScT as compared to conventional metallic DES7-9.
Efforts are ongoing to develop a second generation of safer bioresorbable coronary scaffolds. The hope is that, after absorption and integration processes are complete, event rates will be lower than those of DES. An analytic Markov model of the most recent updated corresponding meta-analyses of randomised clinical trials with Absorb BVS versus metallic stents, performed under the assumption of no scaffold thrombosis or TLR between three and 25 years, suggested that the observed three-year increased rate of ScT would be offset 19 years after PCI10. This means that the allowable excess risk of ScT during the first three years after scaffold implantation must decline significantly in order to justify the value of treatment with a bioresorbable scaffold.
Optimised scaffold implantation techniques have been suggested to mitigate the risk of early and late ScT11. However, serial OCT examples from the INVEST registry demonstrated that ScT also occurs in scaffolds with initially well apposed and well covered struts. This implies that additional non-procedural factors (such as intraluminal late scaffold disintegration) may contribute to the occurrence of ScT12,13. Due to the complex process of scaffold degradation and coronary vessel healing, the exact cause of the reported higher rates of (very) late ScT remain partly understood. Early ScT is most often caused by scaffold underexpansion, and late ScT is caused by scaffold malapposition, either pre-existent or acquired14.
After publication of the preliminary results of the AIDA trial, the steering committee and the Dutch Society of Cardiology advised considering restarting or prolonging DAPT for up to three years after scaffold implantation. In AIDA, twelve of thirteen cases of very late definite ScT occurred in patients who did not take DAPT at the time of the event (Supplementary Table 5). It is still not known whether DAPT is able to mitigate the risk of (very) late ScT. Full three-year follow-up of AIDA might shed light on this issue. Whether the risk of ScT disappears after three years remains to be seen. The four-year results of the ABSORB II trial were encouraging, with no additional scaffold thromboses reported beyond three-year follow-up15. However, in AIDA we observed one case of definite ScT at 1,277 days. We note that, currently, 63.6% of AIDA patients were randomised more than three years ago, but follow-up of these patients is limited. Long-term follow-up of AIDA will provide insights into the long-term risk of ScT beyond the expected time of scaffold resorption and integration (at three and five years, respectively). These insights could be useful for the development of future-generation bioresorbable coronary devices with broader expansion limits, better tensile strength and a more optimal resorption process.
Limitations
First, intracoronary imaging was not performed routinely. It is therefore not possible to distinguish between successful and unsuccessful lesion preparation and/or scaffold implantation. Second, post-procedural cardiac enzymes were only measured when clinically indicated. Therefore, potential post-procedural myocardial infarctions could have been missed. Third, restarting or prolonging DAPT therapy up to three years after scaffold implantation was recommended at the request of the DSMB. This recommendation might have influenced the occurrence of thrombosis-related outcomes in patients on prolonged or restarted DAPT compared to patients who were treated according to the applicable guidelines and IFU.
Conclusions
AIDA formally met its criterion for non-inferiority of Absorb BVS versus XIENCE EES in terms of the combined endpoint of TVF. The Absorb BVS, however, was associated with higher rates of scaffold thrombosis and target vessel myocardial infarction at complete two-year follow-up.
| Impact on daily practice Coronary bioresorbable vascular scaffolds were developed in order to overcome the shortcomings of conventional coronary metallic drug-eluting stents (DES). In AIDA, as in other trials, Absorb BVS was associated with higher rates of scaffold thrombosis and target vessel myocardial infarction than XIENCE EES. In spite of this, AIDA formally met its criterion for non-inferiority of Absorb BVS versus XIENCE EES in terms of TVF. Although Absorb is not available anymore, long-term follow-up of AIDA will provide insights into the long-term risk of scaffold thrombosis beyond the expected time of scaffold resorption and integration (at three and five years, respectively). These insights could be useful for the development of future-generation bioresorbable coronary devices with broader expansion limits and better tensile strength. |
Acknowledgement
The AIDA study team is immensely grateful to Ineke Verhulst and all AMC interventional cardiology research fellows for their continuous support and assistance during the AIDA trial.
Funding
The AIDA trial was supported by an unrestricted educational grant from Abbott Vascular. The AMC Heart Center received an educational research grant from Abbott Vascular for the AIDA trial. The Research Department of the cardiology division of the Medical Center Leeuwarden received non-study-related unrestricted educational research grants from Abbott Vascular.
Conflict of interest statement
J. Piek is a member of the Medical Advisory Board of Abbott Vascular. J. Tijssen served on the DSMB of the early ABSORB trials, including ABSORB II. J. Henriques receives research grants from Abbott Vascular. J. Wykrzykowska receives consultancy fees and research grants from Abbott Vascular. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. Flow chart of patients included in the AIDA trial.
Supplementary Figure 2. 30-day landmark analysis with Kaplan-Meier curves for definite device thrombosis or definite and probable device thrombosis.
Supplementary Figure 3. Subgroup analysis of definite or probable scaffold thrombosis at complete two-year follow-up.
Supplementary Table 1. Characteristics of the treated lesions at baseline.
Supplementary Table 2. Descriptive characteristics of cases of definite device thrombosis.
Supplementary Table 3. Safety and efficacy outcomes at one-year follow-up.
Supplementary Table 4. Safety and efficacy outcomes between one- and two-year follow-up.
Supplementary Table 5. Safety and efficacy outcomes of the “as treated” population at two-year follow-up.
Supplementary Table 6. Safety and efficacy outcomes per protocol treatment at two-year follow-up.
To read the full content of this article, please download the PDF.

