
Introduction
We conducted the AIDA trial comparing the Absorb™ bioresorbable vascular scaffold (BVS; Abbott Vascular, Santa Clara, CA, USA) with the XIENCE everolimus-eluting stent (EES; Abbott Vascular) in daily practice to assess the complete safety and efficacy of the Absorb throughout the scaffold bioresorption period. Preclinical studies have shown that scaffold bioresorption takes 36 months1. Clinical studies have demonstrated that this period of scaffold bioresorption is associated with higher rates of device thrombosis2. Therefore, we herein report the complete three-year clinical outcomes of the Absorb in comparison with the XIENCE.
Methods
The study design, study population and endpoint definitions have been reported in detail previously3,4. All major adverse events were adjudicated by an independent clinical events committee. Time-to-event curves were constructed using the Kaplan-Meier method, and compared by log-rank test. Hazard ratios were determined using Cox regression.
Results
Baseline patient, lesion and procedural characteristics have been described previously4,5. Clinical status at three-year follow-up was known in 97.7% of patients.
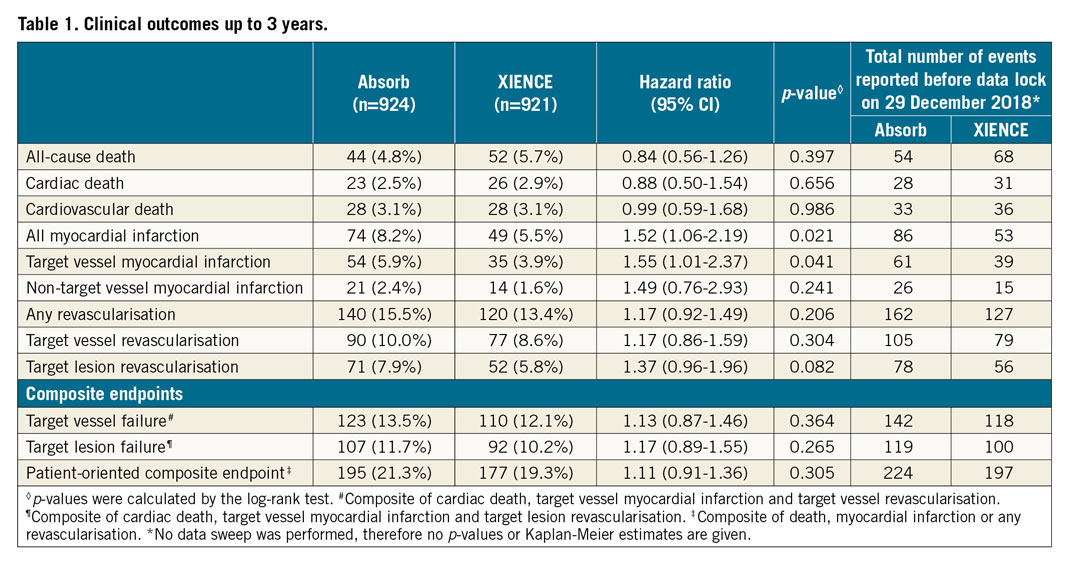
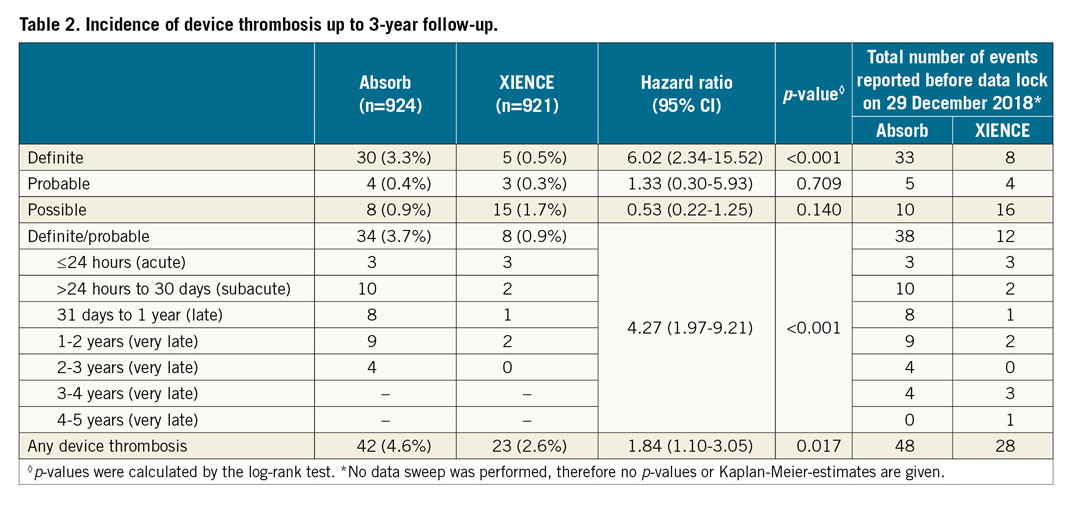
Target vessel failure, cardiac death and target vessel revascularisation continued to accrue at similar rates up to three years in both arms (Figure 1A, Figure 1B). The incidence of target vessel myocardial infarction (TV-MI) was significantly higher in the Absorb arm compared to the XIENCE arm (Table 1, Figure 1C). At three years, 30 patients had definite scaffold thrombosis and five patients had definite stent thrombosis (Table 2, Figure 1D). No case of additional stent thrombosis was noted between two and three years, against four cases of additional scaffold thrombosis. Of note, only one very late definite scaffold thrombosis (VLST) occurred in a patient on dual antiplatelet therapy (DAPT); the other patients with VLST were on single antiplatelet therapy (Supplementary Table 1).
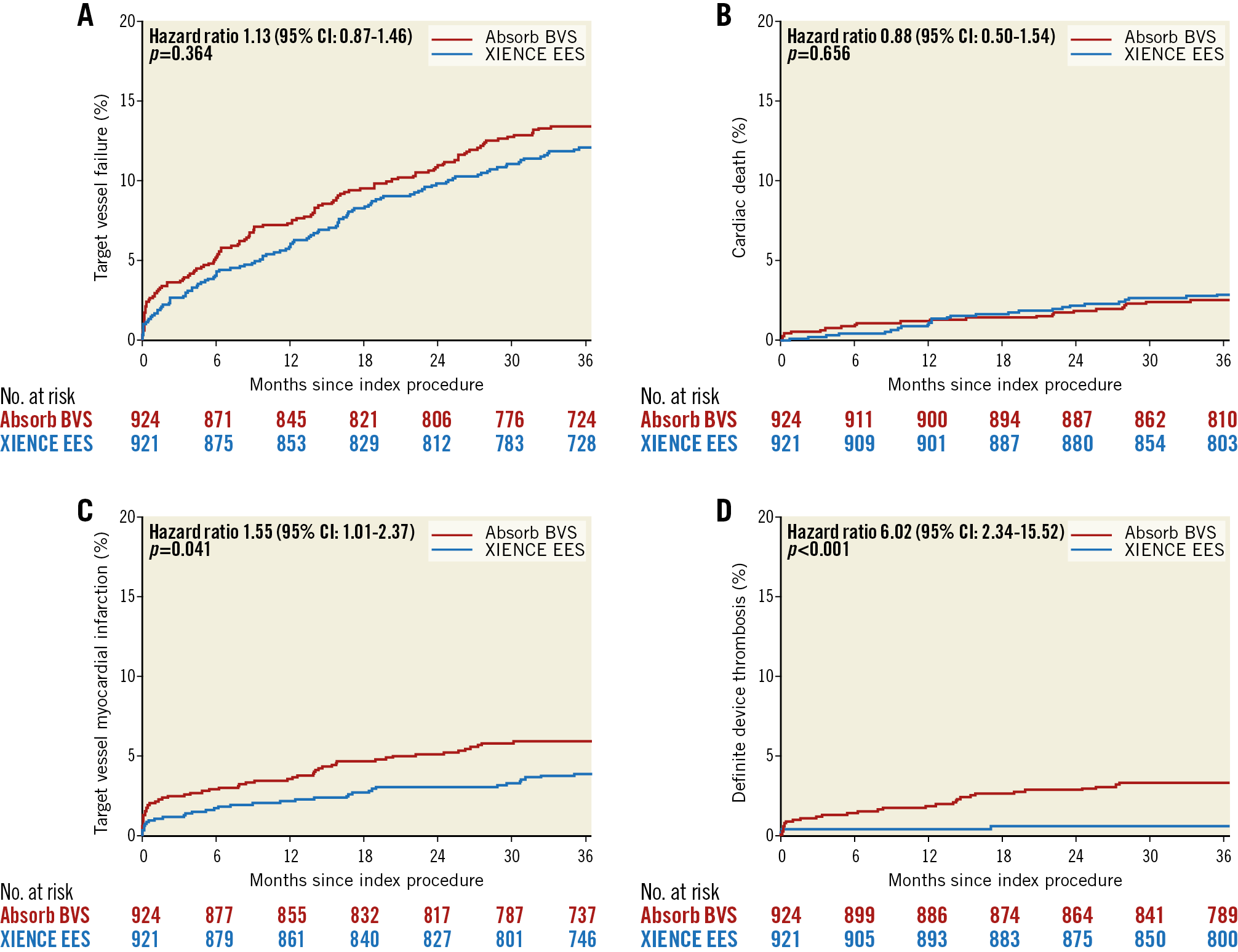
Figure 1. Time-to-first event curves. A) Target vessel failure. B) Cardiac death. C) TV-MI. D) Definite device thrombosis up to three years.
Discussion
AIDA is the largest randomised trial comparing the Absorb BVS to the XIENCE EES in daily practice. At three years, significantly higher rates of TV-MI and definite device thrombosis were seen in the Absorb arm. There was only one VLST in an Absorb-treated patient who continued on DAPT up to three years. The other patients with VLST were on single antiplatelet therapy. The three-year point after Absorb implantation is an important landmark as three years is the approximate period of scaffold polymer absorption1. Intraluminal scaffold dismantling and the bioresorption process are possibly underlying mechanisms of VLST6. Traces of scaffold have been seen beyond three years; whether DAPT should be continued after three years is still uncertain.
Limitations
The lack of systematic intravascular imaging precludes more definite conclusions about the mechanisms related to Absorb failure at different time points. Restarting or prolonging DAPT up to three years after scaffold implantation was recommended at the request of the data safety monitoring board. This recommendation might have influenced the occurrence of thrombosis-related outcomes.
Conclusions
Target vessel failure continued to accrue up to three years in both Absorb and XIENCE. However, the Absorb was associated with higher rates of TV-MI and definite scaffold thrombosis. Long-term follow-up is necessary to examine whether the annual rates of device-related events will decline in Absorb-treated patients after scaffold bioresorption.
|
Impact on daily practice As in other trials, Absorb continued to demonstrate higher rates of scaffold thrombosis and TV-MI compared to XIENCE up to three-year follow-up. Longer-term follow-up of AIDA will provide insights into the long-term safety and potential benefit of Absorb and whether patients treated with Absorb should continue using DAPT. |
Appendix. Study collaborators
Alexander IJsselmuiden, MD; Floris Kauer, MD, PhD; Department of Cardiology, Albert Schweitzer Hospital, Dordrecht, the Netherlands. Marcel Beijk, MD, PhD; Marije Vis, MD, PhD; Karel Koch, MD, PhD; Amsterdam UMC, Heart Center; Department of Clinical and Experimental Cardiology, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands.
Acknowledgements
The authors thank E. McFadden and H.M. Garcia-Garcia for their work in adjudicating all the clinical events.
Funding
The Amsterdam UMC Heart Center received an unrestricted educational research grant from Abbott Vascular for the AIDA trial. The Research Department of the cardiology division of the Medical Center Leeuwarden received non-study-related unrestricted educational research grants from Abbott Vascular.
Conflict of interest statement
J. Tijssen served on the DSMB of the early ABSORB trials, including ABSORB II. J. Henriques received research grants from Abbott Vascular. J. Wykrzykowska received consultancy fees and research grants from Abbott Vascular. The other authors and collaborators have no conflicts of interest to declare.

