
Abstract
Aims: We sought to compare the outcomes of low/moderate complexity patients treated with the Absorb BVS from the ABSORB EXTEND trial with patients treated with the XIENCE everolimus-eluting stent (EES), using propensity score (PS) matching of pooled data from the SPIRIT trials (SPIRIT II, SPIRIT III, SPIRIT IV) and the XIENCE V USA trial.
Methods and results: ABSORB EXTEND was a prospective, single-arm, open-label clinical study in which 812 patients were enrolled at 56 sites. This study allowed the treatment of lesions ≤28 mm in length and with a reference vessel diameter of 2.0-3.8 mm (as assessed by online QCA). The propensity score was obtained by fitting a logistic regression model with the cohort indicator as the binary outcome and other variables as the predictor variables. At one-year clinical follow-up, there was no statistical difference between groups with regard to MACE (5.0% vs. 4.8%, p=0.83), target lesion failure (5.0% vs. 4.7%, p=0.74), ischaemia-driven target vessel revascularisation (2.3% vs. 3.0%, p=0.38) and device thrombosis (1.0% vs. 0.3%, p=0.11). Myocardial infarction was higher with Absorb (3.3% vs. 1.5%, p=0.02), at the expense of periprocedural CK-MB elevation. Independent predictors of MACE among patients receiving Absorb BVS were treatment of multivessel disease, insulin-dependent diabetes and performance of post-dilation.
Conclusions: At one-year follow-up, propensity score-matched analysis demonstrated that the clinical safety and effectiveness of Absorb are comparable to those of XIENCE EES among non-complex patients treated with PCI.
Introduction
Drug-eluting stents (DES) have been shown to reduce markedly the need for repeat revascularisation when compared with bare metal stents and therefore they have become the default devices for percutaneous coronary revascularisation in the past decade1. However, the permanent presence of a metallic device and durable polymer inside the coronary artery might preclude the natural healing process of the vessel, resulting in sustained local inflammatory response and untoward clinical outcomes.
Recently, bioresorbable vascular scaffolds (BVS) have become an attractive alternative to metallic stents, as the need for mechanical support for the healing artery is temporary and, beyond the first few months, there are potential disadvantages of permanent metallic prostheses. However, initial clinical data on their safety are still controversial, with some “real-world” registries suggesting that BVS are associated with a higher incidence of device thrombosis2,3, while others have reported low rates of stent thrombosis (ST)4,5, comparable to those currently observed with second-generation metallic DES. To date, there are six published randomised trials comparing BVS to metallic DES (ABSORB II, EVERBIO II, ABSORB Japan, TROFI II, ABSORB China and ABSORB III)6-11 and all but the ABSORB III have enrolled a relatively low number of patients.
We sought to compare the outcomes of patients with low/moderate complexity coronary artery disease treated with the Absorb BVS (Abbott Vascular, Santa Clara, CA, USA) from the ABSORB EXTEND trial with patients treated with the XIENCE everolimus-eluting stent (EES). We used propensity score (PS) matching of pooled data from the SPIRIT trials (SPIRIT II, SPIRIT III, the non-complex patients from the SPIRIT IV clinical trial) and the near on-label subset of the XIENCE V USA trial.
Methods
STUDY POPULATION
In the present analysis, we included all patients enrolled in the ABSORB EXTEND trial. The details of this study have been described elsewhere12. In brief, the EXTEND study was a prospective, single-arm, open-label clinical trial which enrolled 812 patients at 56 international sites outside the USA. Patients were eligible if they were ≥18 years with evidence of myocardial ischaemia (e.g., stable or unstable angina, silent ischaemia, positive functional study or a reversible change in the 12-lead electrocardiogram [ECG] consistent with ischaemia). Target vessels should have had a reference vessel diameter (RVD) ≥2.0 mm and ≤3.8 mm, a maximum lesion length of ≤28 mm, a diameter stenosis ≥50% and <100% and a Thrombolysis In Myocardial Infarction (TIMI) flow grade of ≥1. A maximum of two de novo native coronary artery lesions could be treated, each located in a different major epicardial vessel. Major exclusion criteria included presentation with recent myocardial infarction (<72 hours to the index procedure), and target lesions located in the left main or within an arterial or saphenous vein graft. Also excluded were lesions with excessive tortuosity and/or heavy calcification.
For the purpose of comparison, the entire ABSORB EXTEND population was compared to patients treated with the XIENCE V® EES (Abbott Vascular) derived from the SPIRIT II, III and IV and XIENCE V USA trials13-16. The details of the SPIRIT trials have been described previously. Of note, major inclusion and exclusion criteria of these trials were similar to those of the ABSORB EXTEND trial, whereas inclusion criteria of the SPIRIT IV trial were more liberal than the other trials, allowing inclusion of patients with complex lesions (defined as a maximum of three target lesions in three separate major epicardial coronary arteries, a maximum of two target lesions in a single coronary artery, an ostial right coronary artery lesion, or bifurcation lesions in which the side branch was ≥2 mm in diameter or the ostium of the side branch had a >50% stenosis).
SOURCE DOCUMENT VERIFICATION
Source document verification (SDV) was routinely performed in 100% of all sites assessing serious adverse events, cardiac events, and related or possibly related (device- or procedure-related) events and in 100% of patients up to 30-day follow-up. Additional SDV was performed at sites with low adverse event reporting. Subsequently, SDV was performed in a random 20% of patients for the remaining follow-up visits.
FOLLOW-UP
Data collection of adverse events, details of any subsequent coronary intervention, and use of and changes in concomitant medications was carried out at 30 days (±7 days), 180 days (±14 days) and one year.
STUDY ENDPOINTS AND DEFINITIONS
The main endpoints of the present analysis included the composite rates of major adverse cardiac events (MACE): cardiac death, myocardial infarction (MI), target vessel failure (TVF), target lesion revascularisation (TLR) and device thrombosis.
An independent clinical events committee (CEC) adjudicated all study endpoints according to either protocol definitions and/or the Academic Research Consortium (ARC) definitions. All adverse events were reported to an independent data and safety monitoring board (DSMB), which reviewed the data to identify safety issues related to the conduct of the study.
Cardiac death was defined as any death due to a proximate cardiac cause (e.g., MI, low-output failure, fatal arrhythmia). Unwitnessed death and death of unknown cause were classified as cardiac death. This comprised all procedure-related deaths including those related to concomitant treatment.
The MI classification and criteria for diagnosis were defined according to the per-protocol definition; Q-wave MI was the development of a new, pathological Q-wave. Non-QMI was elevation of CK levels to ≥2 times the upper limit of normal with elevated CK-MB in the absence of new pathological Q-waves.
Ischaemia-driven target vessel failure (ID-TVF) was composed of cardiac death, myocardial infarction (Q-wave and non-Q-wave) and ischaemia-driven target vessel revascularisation by CABG or PCI.
Ischaemia-driven target lesion revascularisation (ID-TLR) was defined as any repeat percutaneous intervention of the target lesion or bypass surgery of the target vessel with either positive functional ischaemia study, ischaemic symptoms and angiographic minimal lumen diameter stenosis ≥50% by core laboratory QCA, or revascularisation of a target lesion with diameter stenosis ≥70% by core laboratory QCA without either ischaemic symptoms or a positive functional study.
Device thrombosis was categorised as acute (<1 day), subacute (1-30 days) and late (>30 days) and was defined according to the ARC guidelines (definite: acute coronary syndrome and angiographic or pathologic confirmation of stent/scaffold thrombosis; probable: unexplained death ≤30 days or TV-MI without angiographic confirmation of stent/scaffold thrombosis)17.
The ABSORB EXTEND and all trials used in the comparative analysis were sponsored and funded by Abbott Vascular. The research ethics committee of each participating institution approved the protocol. All enrolled patients provided written informed consent before inclusion.
STATISTICAL ANALYSIS
For the descriptive statistics, categorical data are presented as counts and percentages and continuous variables are presented as mean±standard deviation (SD). Categorical variables were compared by the chi-square test or Fisher’s exact test when Cochran’s rule was not met. Continuous variables were compared using the Student’s t-test.
Propensity score matching was applied to compare one-year clinical outcomes of patients treated with Absorb BVS and those treated with XIENCE EES. The propensity score was obtained by fitting a logistic regression model with the cohort indicator as the binary outcome, and other variables (shown as follows) as the predictor variables: age, gender, current smoker, hypertension requiring medication, hypercholesterolaemia requiring medication, diabetes treated with insulin, family history of CAD, unstable angina, prior coronary intervention, prior MI, multivessel disease, at least one B2/C lesion, long lesion length (mm), small reference vessel diameter (RVD) (mm), and greater diameter stenosis (DS) (%).
Once the propensity score was obtained, patients in the two groups were matched through a greedy algorithm based on local optimisation. The control selected for a particular case should be the one closest to the case in terms of distance, whereby the maximum allowed distance for matching was set to 0.10 in the present study. Analyses were then performed on the two groups for the matched patients, treating them as two independent samples. A 1:1 matching was used in this analysis.
To determine the independent predictors of MACE, TLR, MI and thrombosis among patients treated with Absorb BVS and XIENCE V, a multivariable logistic regression model was built using a stepwise (forward/backward) procedure, with independent variables entered into the model at the 0.20 significance level and removed at the 0.10 level. Variables were eligible for inclusion in the multivariable logistic regression model-building process if the variable was present for 90% of the subjects in the analyses, they had a p-value <0.2 from the univariable analysis, and, if highly correlated with another variable (r>0.5 and p<0.05), they had the higher level of significance. Time-to-event variables are presented as Kaplan-Meier curves. A two-tailed p-value <0.05 was considered statistically significant.
Results
BASELINE POPULATION AND ANGIOGRAPHIC CHARACTERISTICS
A total of 812 patients were enrolled in the ABSORB EXTEND single-arm trial between January 2010 and October 2013. The entire cohort was matched to patients from the SPIRIT trials (n=812) and a 1:1 matching was performed. As shown in Table 1, both cohorts were very similar at baseline, with the exception of family history of CAD, which was more prevalent among patients treated with XIENCE EES (42% vs. 37%, p=0.04). Overall, patients were relatively young (~61 years old) and predominantly male (>70% in both groups). Diabetes mellitus was observed in almost 27% of all patients but the rate of insulin dependence was relatively low (~5%). Stable angina was prevalent in both cohorts.
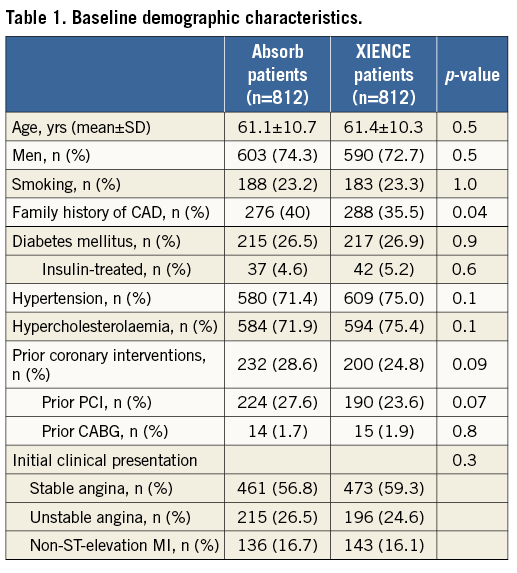
Table 2 displays the most relevant angiographic findings for both groups. Single lesion/vessel treatment was the most frequent finding in both groups (>90%, p=0.2), while the percentage of patients with at least one B2/C lesion corresponded to barely half of the population in both groups (46.5% for Absorb BVS vs. 48.2% for XIENCE EES, p=0.5). As per protocol, predilatation was performed in almost all cases in the ABSORB EXTEND patients (99.7% vs. 69.4%, p<0.001). Similarly, post-dilation was performed more often after Absorb BVS than after XIENCE EES implantation (68.8% vs. 42.2%, p<0.001). Mean lesion length of the longest lesion treated was slightly longer in the XIENCE EES cohort (12.5±5.3 mm vs. 13.3±5.6 mm, p=0.003); however, device length was equivalent in both groups (23.4±9.8 mm vs. 23.8±12.1 mm, p=0.5). Average RVD was smaller in the ABSORB EXTEND population (2.64±0.39 mm vs. 2.69±0.44 mm, p=0.01).
Clinical events
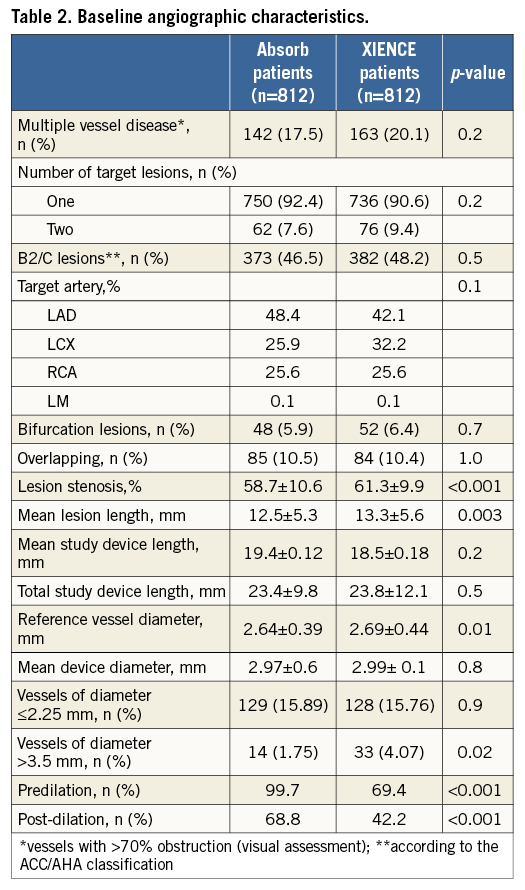
One-year clinical follow-up was available for all patients. Table 3 shows the incidence of clinical adverse events at one year. As noted, the incidence of hierarchical MACE (5.0% vs. 4.8%, p=0.83) and TVF (5.5% vs. 6.2%, p=0.57) was low and no statistical difference was seen between the cohorts. While the incidence of cardiac death and TLR was comparable in the two groups, the occurrence of MI was significantly higher in the ABSORB EXTEND cohort (3.3% vs. 1.5%, p=0.02), mainly at the expense of periprocedural MI (2.7% in the ABSORB cohort vs. 1.4% in the XIENCE group, p=0.06). There was also a non-significant higher occurrence of definite/probable device thrombosis (1.0% vs. 0.3%, p=0.11).
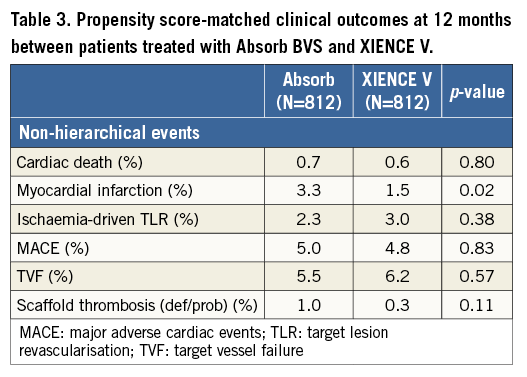
Figure 1 shows the cumulative incidence of MACE (Figure 1A), MI (Figure 1B), TLR (Figure 1C) and definite/probable device thrombosis (Figure 1D) in the two groups. As noted, the higher incidence of MI in the ABSORB EXTEND cohort was mainly driven by in-hospital periprocedural MI, while most scaffold thrombosis occurred within the first six months after PCI (Figure 2).
Figure 1. Cumulative incidence of MACE, MI, TLR and definite stent thrombosis. A) MACE. B) MI. C) TLR. D) Definite stent thrombosis.
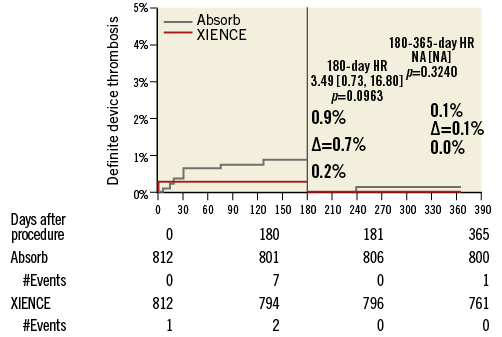
Figure 2. Landmark analysis of definite device thrombosis. Notably, the vast majority of Absorb definite thrombosis occurred within six months of the index procedure.
MULTIVARIATE ANALYSIS
Independent predictors of MACE among patients treated with the Absorb BVS included treatment of multiple lesions (OR 3.09, 95% CI: 1.32-7.24, p=0.009), need for bail-out scaffold (OR 4.27, 95% CI: 1.58-11.57, p=0.004), treatment of insulin-dependent diabetics (OR 3.10, 95% CI: 1.06-9.10, p=0.04) and post-dilation (OR 2.51, 95% CI: 1.07-5.90, p=0.03).
Regarding the occurrence of MI, the independent predictors were treatment of patients with a previous history of MI (OR 2.96, 95% CI: 1.35-6.49, p=0.007), treatment of multiple lesions (OR 1.34, 95% CI: 1.1-2.95, p=0.041) and the use of more than one scaffold/lesion (OR 3.84, 95% CI: 1.64-8.96, p=0.02).
In the XIENCE V cohort, the independent predictors of MACE were treatment of insulin-dependent diabetics (OR 4.03, 95% CI: 1.5-10.9, p=0.006) and treatment of ostial lesions (OR 5.6, 95% CI: 1.3-25.0, p=0.02). There were no independent predictors of MI in the XIENCE V cohort.
Since cardiac death, TLR and scaffold thrombosis were low-frequency events in both groups, it was not possible to estimate their independent predictors with reasonable accuracy.
Discussion
The current study shows equivalence in the performance of Absorb BVS and metallic EES in the treatment of patients with coronary disease of low-to-moderate complexity up to one-year clinical follow-up. Overall, the incidence of events was relatively low and more prevalent in the first six months after PCI, especially in the BVS cohort.
Despite not being randomised, the findings of our study are aligned with the results of the previous randomised trials. In the ABSORB II trial, a randomised, multicentre trial with 501 patients treated with either Absorb BVS or metallic EES (2:1 randomisation), the MACE rate was 5.1% vs. 3.0%, respectively. The incidence of MI was also slightly higher in the BVS cohort of that trial (4.0% vs. 1.0%, p=0.06)6. In the EVERBIO II trial (n=320), a randomised, single-centre evaluation of the Absorb BVS versus two contemporary metallic DES in “all comers”, the rate of the device-oriented composite endpoint (cardiac death, MI related to target vessel and TLR) was 12% with the Absorb BVS versus 9.0% with the metallic DES (p=0.6)7. In the ABSORB Japan trial, the TLF rates were 4.2% vs. 3.8% for BVS vs. CoCr EES, p=0.858. Finally, in the ABSORB III trial, the largest randomised trial to date, TLF occurred in 7.8% of the patients in the Absorb cohort and 6.1% in the XIENCE group (pnon-inferiority=0.007) with an absolute difference of 0.8% in the device thrombosis rate (0.7% in the XIENCE group vs. 1.5% in the Absorb cohort, p=0.13) at one-year follow-up. These findings were corroborated by a recently published meta-analysis comparing XIENCE to Absorb18.
Regarding scaffold thrombosis, randomised trials have not shown a significant difference in the incidence of this untoward event between patients treated with metallic second-generation DES and Absorb, despite a trend to higher numerical events with the scaffold, mainly within the first days/months after the index procedure. This finding was also corroborated by the previously mentioned meta-analysis18. In addition, Brugaletta et al recently published the comparison of metallic stents (DES and BMS) versus Absorb in the scenario of ST-elevation MI. In their analysis, most of the thrombosis in the Absorb cohort occurred within 30 days of the procedure, with a statistical trend favouring the metallic devices (2.1% with Absorb vs. 0.3% with XIENCE, p=0.059; vs. 1.0% with BMS, p=0.324)19. Similarly, in the present study, the majority of the scaffold thrombosis occurred in the early phase after PCI, with very few cases reported after six months.
The higher incidence of MI in the BVS group might be related to the definition of periprocedural MI, device characteristics (increased strut thickness and width, higher scaffold-to-artery coverage ratio) and deployment technique, which requires more “aggressive” lesion preparation. Recently, Kawamoto et al analysed 499 patients treated with either Absorb BVS or first-generation CYPHER® DES (Cordis, Johnson & Johnson, Miami Lakes, FL, USA) and found a higher rate of periprocedural MI (BVS 13.1% vs. SES 7.5%, p=0.05)20. This is an interesting finding considering that both devices have similar strut thickness, and may be explained by the higher strut surface area of the BVS. In the ABSORB II trial, deployment of BVS resulted in a trend towards a higher rate of side branch occlusion as compared to the metallic EES (Absorb 5.3% vs. XIENCE 7.6%, p=0.07)6, an event that similarly may be due to the higher BVS footprint.
Lesion preparation is a crucial step in BVS implantation. In the current stage of BVS development, predilatation is strongly recommended in order not only to facilitate scaffold deliverability but also to favour its adequate expansion and apposition. Predilation might also help to size the vessel properly, guiding correct device selection. Conversely, direct stenting might reduce vessel wall damage and distal embolisation. Indeed, previous studies showed a correlation between predilatation and increased periprocedural CK-MB release21. Notably, this finding does not seem to correlate with a significant increase in mortality, the occurrence of spontaneous MI, TLR or device thrombosis.
Additionally, the rate of scaffold overlapping in our study (~10%) seems to be higher than previously reported in other BVS evaluations. Overlapping of Absorb BVS results in a segment with a 300 to 400 μm layer of polymer that might impair local flow dynamics and also increase the risk of side branch occlusion. In this regard, a recently published post hoc analysis of the ABSORB II trial showed that BVS overlapping is an independent predictor of CK-MB release (OR 5.07, 95% CI: 1.78-14.41, p=0.002)22. In our study, bail-out scaffold use and treatment of more than one lesion per patient were independent predictors of MACE and MI among patients treated with Absorb BVS, corroborating the above findings.
Finally, post-dilatation was a predictor of MACE among patients treated with Absorb in our study. This finding must be interpreted with caution. Indeed, post-dilatation has become very frequent in contemporary practice after Colombo et al demonstrated that dilating Palmaz-Schatz stents at high pressures improved their expansion and apposition, reducing acute/subacute thrombotic events and eliminating the need for anticoagulation23. Afterwards, several studies inversely correlated final stent dimensions with the occurrence of thrombosis and restenosis. Therefore, the “bigger is better” concept became a mantra of modern interventional cardiology. Conversely, post-dilation is associated with more distal embolisation and flow disturbance phenomena, especially in the setting of acute coronary syndromes. Our group has previously published on the impact of post-dilation on patient outcome after Absorb BVS implantation. Among 768 patients, post-dilation was associated with a numerically threefold higher incidence of MI as compared with patients without post-dilation (4.2% vs. 1.6%, p=0.6). However, as in the present study, this had no clinical impact in terms of death, TLR or scaffold thrombosis24.
The current recommendation, based on bench tests, preclinical and clinical reports, is that post-dilation of the Absorb BVS, whenever necessary to optimise acute angiographic results, should be performed. However, a non-compliant balloon catheter not more than 0.5 mm greater than the implanted scaffold diameter (e.g., a non-compliant balloon with a maximum size of 3.5 mm for a 3.0 mm scaffold) should be used at nominal pressure for post-dilation. Ideally, proper vessel sizing and good lesion preparation may help to ensure that excessive post-dilatation is rarely required.
Limitations
This study was limited by the single-arm nature of the design and the inherent lack of a control arm for direct comparison. Even though propensity matching was used, it is still possible that there were unadjusted confounders (particularly related to lesion characteristics), which may have influenced the results. Second, we did not use the universal definition of MI due to the absence of data on troponin in the XIENCE EES group. The definition of MI was the same in both the ABSORB EXTEND and SPIRIT trials.
Conclusions
Twelve-month clinical results from the full ABSORB EXTEND study cohort demonstrate that treatment of relatively non-complex patients with the Absorb BVS is safe and results in low rates of MACE and scaffold thrombosis. In addition, propensity score-matched analysis demonstrated that the clinical safety and effectiveness of Absorb BVS are comparable to those of the XIENCE V among this population.
| Impact on daily practice ABSORB BVS have been more frequently used in current PCI practice as an alternative to metallic stents to keep immediate vessel patency and later restore normal endothelial function. However, recent publications have raised concerns on the safety and efficacy of this technology in more complex angiographic scenarios. The present research provides reassurance about the similar efficacy of these devices in comparison to a second-generation metallic DES in treating patients with low to moderate complexity CAD. However, as in most randomised trials, a trend to more device thrombosis was observed with these new devices. |
Guest Editor
This paper was guest edited by Antonio Colombo, MD, FACC, FESC, FSCAI; Department of Interventional Cardiology, EMO GVM Centro Cuore Columbus, Milan, Italy.
Funding
All trials used in the comparative analysis were sponsored and funded by Abbott Vascular.
Conflict of interest statement
J. de Ribamar Costa Jr is a proctor for Abbott Vascular. A. Abizaid is a consultant for Abbott Vascular and Reva Medical. M. Stuteville, D. Ediebah, K. Sudhir are all full-time employees of Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

