Abstract
Aims: To report procedural and midterm clinical outcomes after the use of the second-generation Absorb everolimus-eluting bioresorbable vascular scaffold (Absorb BVS) in a real-world percutaneous coronary intervention (PCI) registry.
Methods and results: All patients assigned to treatment with the Absorb BVS in the Academic Medical Center, Amsterdam, between August 2012 and August 2013 were included in a prospective registry. A total of 135 patients were included in the study, including 53 (39%) acute coronary syndrome (ACS) patients (13% ST-segment elevation myocardial infarction [STEMI]). In total 159 lesions were treated, including 102 (62%) with a type B2 or C classification. Pre- and post-procedural quantitative coronary angiography (QCA) analyses showed an acute gain of 1.37±0.53 mm. An angiographic success rate was achieved in 152 (96%) of the lesions. Six-month follow-up was available in 97% of the patients. Six-month cumulative target vessel failure (composite of all-cause mortality, any myocardial infarction [MI] and target vessel revascularisation [TVR]) rate was 8.5%, including a 3.0% MI, 3.0% definite scaffold thrombosis, 6.3% target lesion revascularisation, and an 8.5% TVR rate.
Conclusions: The use of the Absorb BVS in a cohort reflecting daily clinical practice is feasible and associated with good procedural safety and angiographic success rate. In addition, six-month follow-up is associated with acceptable clinical outcomes.
Introduction
After the introduction of coronary balloon angioplasty in 19771, numerous advances in percutaneous coronary intervention (PCI) followed. Although drug-eluting stents (DES) were designed to reduce in-stent neointimal hyperplasia by an antiproliferative drug coating, shortcomings remained, such as late in-stent restenosis and neoatherosclerosis, with subsequent late and very late stent thrombosis2,3. Moreover, permanent caging of the vascular wall is known to cause permanent jailing of the side branch, hinder surgical revascularisation, and impair vasomotion and imaging of the stented segment with coronary computed tomography4. The Absorb bioresorbable vascular scaffold (BVS) (Abbott Vascular, Santa Clara, CA, USA) is a completely resorbable device designed to provide temporary scaffolding and everolimus antiproliferative drug delivery, allowing the vessel wall to heal without leaving a permanent metallic stent behind, and potentially overcomes the limitations of metallic stents5.
The Absorb BVS is the first bioresorbable scaffold approved for clinical use in Europe. Although favourable results were observed in the ABSORB cohort A and B trials for safety and efficiency, the inclusion criteria were highly restricted and limited to the treatment of relatively simple coronary artery lesions6-8. Shortly after the Absorb BVS became commercially available, the Academic Medical Center (AMC) started to implant the scaffold in a “real-world” patient population.
Our registry was conducted to evaluate the procedural success, acute angiographic results and six-month clinical outcomes of the Absorb BVS in a real-world patient population.
Methods
SETTING
This registry was performed in a high-volume, tertiary PCI centre, the AMC Medical Center, University of Amsterdam, The Netherlands, with an annual PCI volume of approximately 2,000 PCI procedures.
In August 2012 interventional cardiologists in the AMC were trained and certified to implant Absorb BVS, which was followed by an increased use in daily clinical practice. In order to prepare the lesion for BVS implantation optimally, operators were instructed to abide by the “instructions for use” (IFU) of the Absorb BVS. These instructions state that predilatation with an angioplasty balloon shorter than the planned scaffold length and 0.5 mm smaller or equal to the scaffold device diameter is recommended. Deployment of the scaffold is recommended with a slow increase of two atmospheres every five seconds until the scaffold is completely expanded. Post-dilatation within the described dilatation limit of the implanted scaffold was allowed at the discretion of the operator. The use of online quantitative coronary angiography (QCA) to assess appropriate device size was at the operator’s discretion. Patients received dual antiplatelet therapy (DAPT) for at least 12 months.
STUDY DEVICE
The Absorb BVS (Abbott Vascular) technology is described in detail elsewhere9. In short, the polymer scaffold is a composite of a poly L-lactide acid (PLLA) backbone coated with a matrix of everolimus and poly D, L-lactide (PDLLA). Two radiopaque platinum markers are located 1.7 mm from the distal and proximal ends of the scaffold to allow visualisation on angiography. The VISION RX delivery system (Abbott Vascular) has two markers on the proximal and distal ends, located 0.5 mm inside the scaffold. Due to the degradation process, the scaffold no longer gives vessel wall support after six to twelve months. The available scaffold lengths were 12, 18 and 28 mm, with scaffold diameters 2.5, 3.0 and 3.5 mm.
STUDY DESIGN
The study population consisted of all patients who underwent PCI with an Absorb BVS implantation between August 30th, 2012, and August 6th, 2013, during routine clinical practice. The decision to implant an Absorb BVS was at the discretion of the operator. Patients with a wide range of indications, ranging from stable angina to acute coronary syndrome, and with a diverse range of lesion characteristics were included, similar to a real-world situation.
Baseline demographic characteristics were prospectively collected. All angiographic films were reviewed and discussed with an interventional cardiologist to obtain procedural and angiographic characteristics. The pre- and post-procedural angiograms were analysed offline with quantitative coronary angiography (QCA) software. Clinical follow-up was obtained by telephone contact. When potential events were reported, this was cross-checked in patients’ medical records, discharge summaries and, when applicable, repeat angiograms were reviewed. If patients could not be contacted, follow-up information regarding vital status was obtained from the national population registry (Dutch Central Bureau of Statistics) and hospital records were obtained from the last medical contact. All reported events were verified and adjudicated by an interventional cardiologist (JJW) according to the criteria defined below.
QUANTITATIVE CORONARY ANGIOGRAPHY
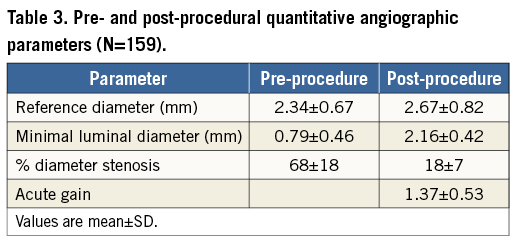
Angiographic films were analysed with dedicated software (QAngioXA version 7.3; Medis, Leiden, The Netherlands) by two of the investigators (RK and MG) and a dedicated bifurcation algorithm was used in cases where the target lesions involved a side branch. Reference vessel diameter, minimal luminal diameter (MLD) and percentage diameter stenosis (%DS) were obtained for the target vessel in the pre-procedural angiographic film. Matched views of immediate post-procedural films were then selected to determine the reference vessel diameter, in-scaffold MLD and in-scaffold %DS. Acute gain was defined as the difference between pre-procedure MLD and post-procedure MLD within the scaffold.
Definitions
Angiographic success was defined as <30% residual stenosis in the target lesion on QCA with Thrombolysis In Myocardial Infarction (TIMI) 3 flow in the intended target vessel. Procedure success was defined as angiographic success in the absence of in-hospital target vessel failure (TVF). TVF was defined as a composite of the device-oriented endpoints of all-cause mortality, any myocardial infarction (MI) and target vessel revascularisation (TVR). TVR was defined as any revascularisation in the target vessel. TLR was defined as any revascularisation within 5 mm distance of the index lesion. MI definitions were in accordance with the most recent universal definition of MI10. Stent thrombosis was defined according to the Academic Research Consortium (ARC)11.
Statistical analysis
Continuous data are expressed as mean±standard deviation (SD) or as median (interquartile ranges) and dichotomous data are summarised as frequencies. Cumulative event rates were estimated using the Kaplan-Meier method. Follow-up was censored at six months or at the last known date of follow-up, whichever came first. Statistical analysis was performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
Results
BASELINE AND LESION CHARACTERISTICS
A total of 135 patients were enrolled and 159 lesions were treated. Baseline and lesion characteristics of patients enrolled are shown in Table 1. Mean age was 59 (±11) years, patients were predominantly male (73%), and 20% were diabetics. Stable angina was the indication for PCI in 47%, and ST-segment elevation myocardial infarction (STEMI) in 13%.
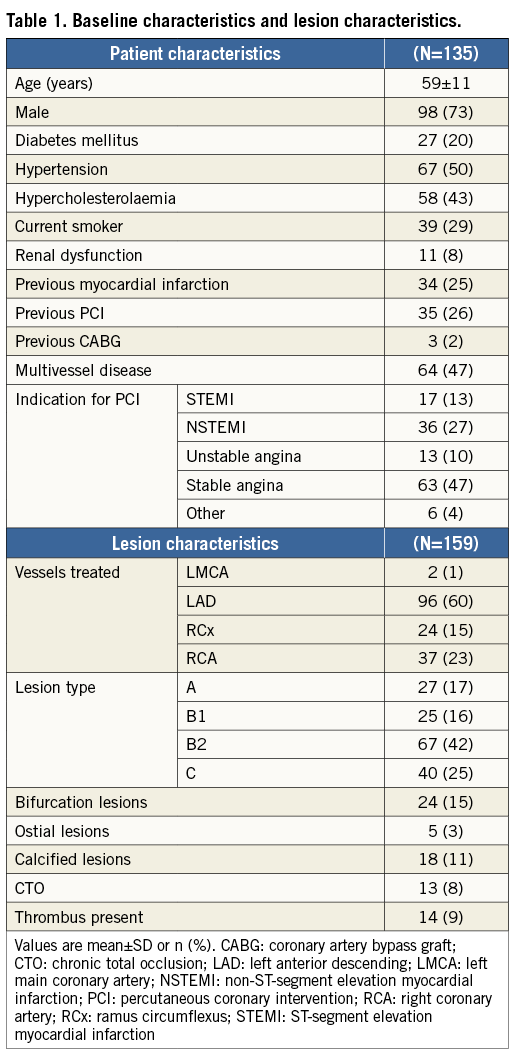
Sixty-four patients (48%) had multivessel disease. Most lesions (67%) were lesion type B2 or C (AHA/ACC classification), including two left main, 13 chronic total occlusions and 24 bifurcation lesions.
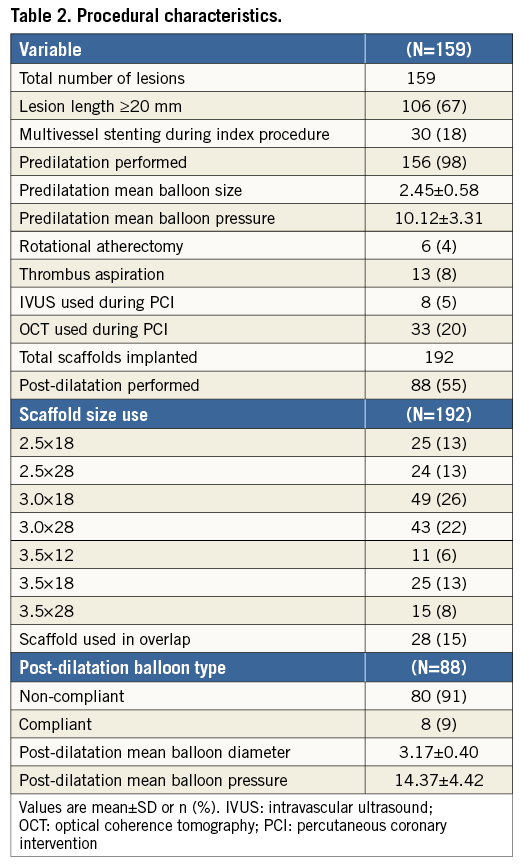
PROCEDURAL CHARACTERISTICS
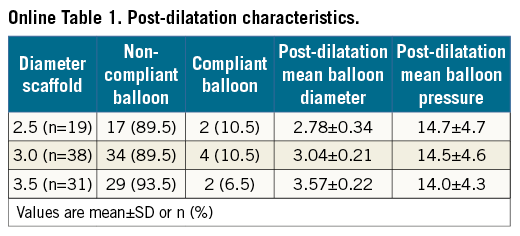
Procedural characteristics are shown in Table 2. Predilatation was performed in 156 (98%) lesions, rotational atherectomy in six (3.8%) lesions and thrombus aspiration in 13 (8.2%) lesions. In total, 192 scaffolds were implanted. Post-dilatation was performed in 88 lesions (55%) (Online Table 1).
PCI of bifurcation lesions was performed with either a single scaffold procedure (Figure 1) or a modified culotte strategy using an Absorb BVS in combination with a dedicated bifurcation Tryton stent (Tryton Medical, Inc., Durham, NC, USA).
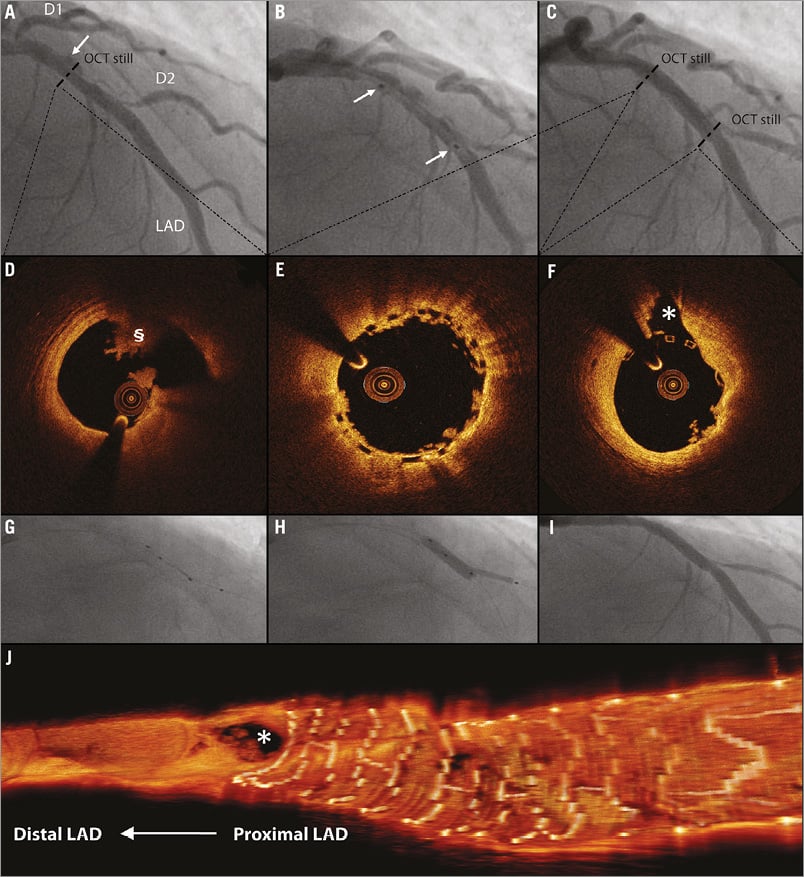
Figure 1. Single scaffold strategy in bifurcation lesion. *indicates second diagonal branch; §indicates abnormal intraluminal tissue (i.e., thrombus). A) A 58-year-old male was admitted to our hospital with STEMI. After thrombus aspiration, the coronary angiogram revealed a suspected ulcus in the LAD. B) After predilatation, a 3.5×18 mm Absorb BVS was deployed in the mid LAD at 16 atm. C) Final angiogram showed an optimal result of the LAD. D) OCT, before BVS placement, showed rupture of a lipid plaque. E) & F) OCT still frames after BVS placement and fenestration in the direction of D2 showed complete coverage of the ruptured plaque and an open SB ostium. Because of severe pinching of the D2 ostium, the BVS was fenestrated in the direction of D2 using a Sprinter wire (Medtronic, Minneapolis, MN, USA). G), H) & I) The procedure was finalised with kissing balloon inflation using 2.5×20 (8 atm) and 3.5×15 (12 atm) Trek NC balloons (Abbott Vascular) in the D2 and LAD, respectively. J) Three-dimensional OCT reconstruction of the final OCT pullback showed scaffold placement proximal to the SB and confirmed an open SB ostium.
QUANTITATIVE CORONARY ANGIOGRAPHY ANALYSES
Baseline QCA analyses were available for all lesions (Table 3). Pre-procedural MLD was 0.79 mm, with a mean %DS of 68. Post-procedural MLD was 2.16 mm, resulting in an acute gain of 1.37 mm. Angiographic success was 96% due to seven lesions with residual stenosis >30% (range 31% to 49%).
Clinical outcomes
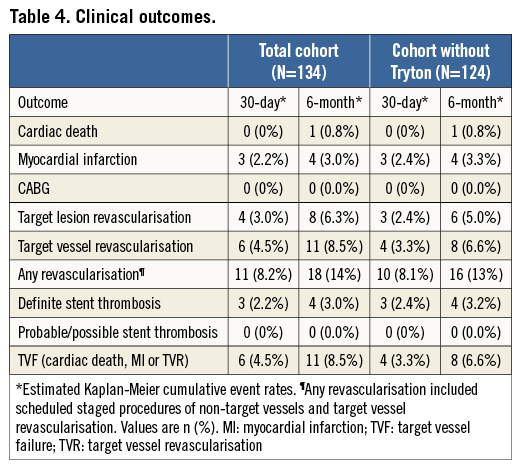
Median follow-up was 198 days (187-209). Thirty-day follow-up was available in 134 (99%) and six-month follow-up was available in 132 (97%) patients. Three patients were lost to follow-up.
One case of cardiac death, following scaffold thrombosis (ST), occurred at six-month follow-up (six-month cumulative event rate 0.8%). Four cases of ST (one in-hospital) occurred which all subsequently resulted in MI (six-month cumulative MI rate of 3.0%). All four cases of ST (cumulative rate of 3.0%) were defined as definite, including three subacute and one late ST. Besides one in-hospital MI/ST, no other in-hospital events occurred (procedural success 95%).
In addition to ST, four additional TLRs occurred (in total eight patients, 6.3%). Two patients were initially treated for a bifurcation lesion with Absorb BVS in combination with a Tryton stent and later returned with recurrent angina. A full description of all TLRs is given in the Online Appendix. TVR occurred in three more patients due to recurrent symptoms. Non-TVR occurred in nine patients (6.8%) and these were all scheduled staged procedures.
TVF occurred in 11 patients (8.5% six-month TVF rate).When excluding patients treated with the Absorb in combination with the Tryton stent (n=124) the six-month TVF rate was 6.6% (Table 4).
Case description of scaffold thrombosis
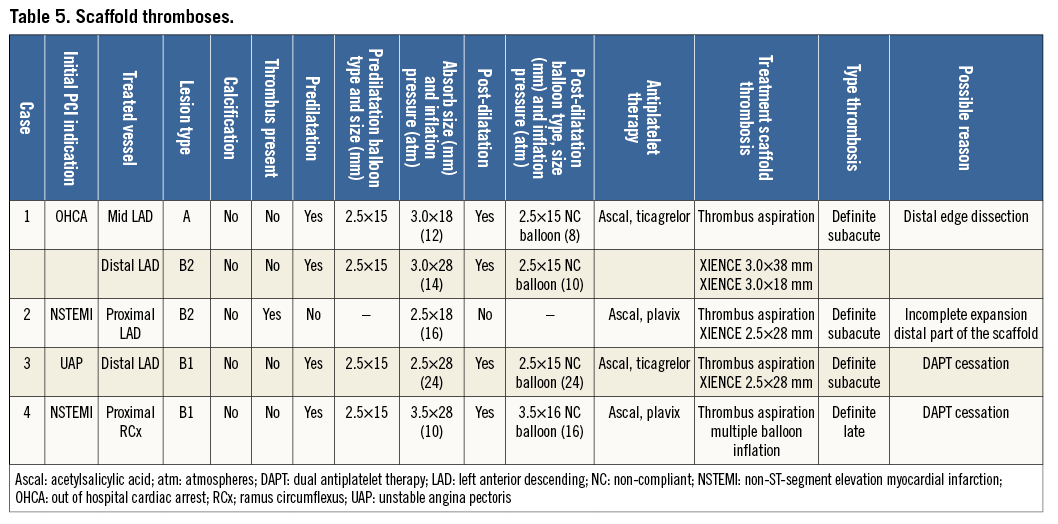
The first patient with ST was a 53-year-old male admitted to the intensive care unit after experiencing an out-of-hospital cardiac arrest and who was subsequently treated semi-electively with implantation of two non-overlapping scaffolds (3.0×18 mm and 3.0×28 mm, distal edge post-dilatation with 2.5×15 mm at 8 atm) in the LAD. One week later the patient experienced an MI. Repeat angiography revealed a filling defect suggestive of thrombosis of the proximal implanted scaffold in the LAD. Thrombus aspiration was performed over a Balance Middleweight (BMW) guidewire (Abbott Vascular) and subsequently a Hi-Torque Pilot 50 hydrophilic guidewire (Abbott Vascular) was advanced into the distal LAD. After guidewire placement, no angioplasty balloon could be advanced, probably because of a false route, and the procedure was stopped. Three days later a repeat procedure was performed. The LAD was predilatated and two XIENCE Xpedition (Abbott Vascular) drug-eluting stents (DES) were implanted within the scaffold. The ST probably resulted from a distal edge dissection which had occurred during the initial procedure.
The second case was a 67-year-old male who initially underwent urgent PCI of a thrombotic occlusion of the mid LAD in the setting of ACS with direct implantation of a 2.5×18 mm BVS. Three days later he experienced recurrent angina with ST-segment elevations in the territory of the BVS-treated coronary artery. Angiography showed a total occlusion of the implanted scaffold. Thrombus aspiration was performed and thrombotic material was retrieved, which was followed by XIENCE Xpedition DES implantation within the BVS. OCT pullback performed before implantation of the XIENCE Xpedition DES showed an incomplete expansion of the distal part of the Absorb BVS as the most likely cause of the ST. OCT after XIENCE implantation showed a fully deployed stent within the previously placed scaffold (Figure 2).
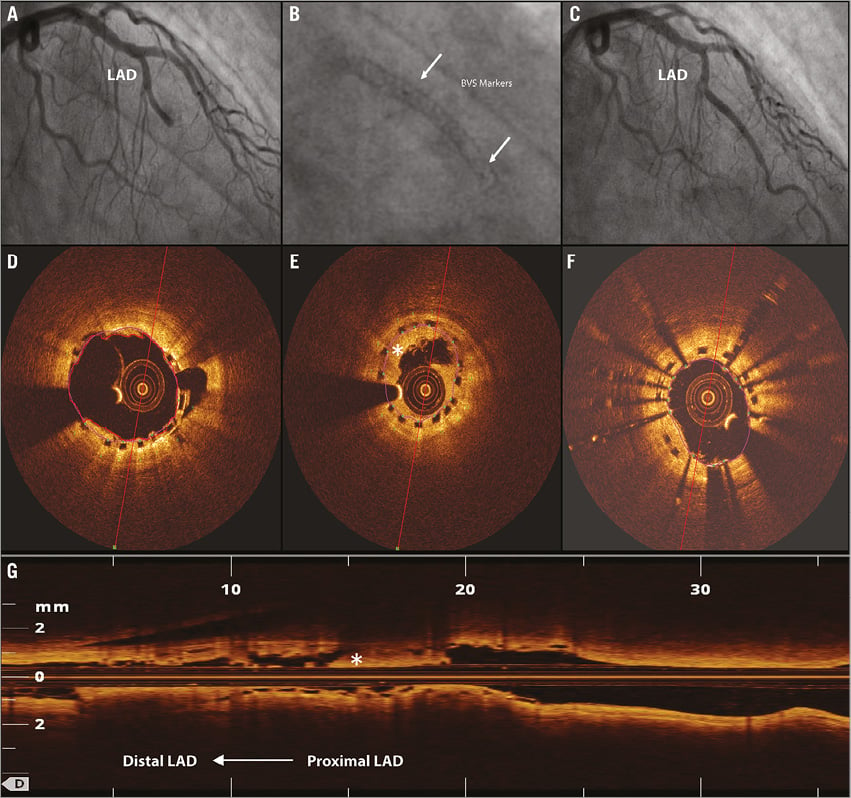
Figure 2. Scaffold thrombosis. *indicates abnormal intraluminal tissue (i.e., thrombus). A 67-year-old male presenting with an acute coronary syndrome was initially treated at our hospital with implantation of a 2.5×18 mm Absorb BVS at 16 atm. Three days after implantation the patient developed recurrent angina and ST-segment elevation in the precordial leads. A) Angiography showed a total occlusion of the previously placed BVS. D) After thrombus aspiration an optical coherence tomography (OCT) pullback was performed which showed full deployment of the proximal part of the scaffold; however, the distal part of the scaffold was underexpanded. E & G) OCT analyses showed a minimal scaffold diameter in the underexpanded part of 1.90 mm. B) A 2.5×28 XIENCE Xpedition drug-eluting stent was implanted within the BVS at 14 atm. F) OCT showed a minimal scaffold diameter of the distal stented segment of 2.23 mm . C) Final angiography showed an optimal result. Implantation of a DES within an incompletely expanded scaffold is debatable; additional balloon dilatations could also probably have been a good treatment option in this case.
The third case was a 55-year-old male, with an extensive history of coronary artery disease including CABG and multiple DES placements, who presented with a NSTEMI 23 days after BVS (2.5×28 mm) implantation in the LAD. In an attempt to commit suicide the patient had deliberately stopped using his DAPT one week prior to the ST. Angiography showed occlusion of the scaffold, which was followed by successful thrombus aspiration and multiple balloon dilatations resulting in restoration of TIMI 3 flow. A dissection of the LAD was seen and scaffold destruction suspected, so no direct stent placement was performed. After five days, a XIENCE DES was implanted within the scaffold.
The fourth case of ST resulted in the death of the patient. Initially, the patient presented with NSTEMI and was treated with a 3.5×28 mm BVS in the mid RCx. Three months later he presented with an acute inferoposterior infarction after DAPT had been discontinued five days earlier because the patient suffered from gastrointestinal bleeding. Angiography showed an occluded BVS, which was treated by thrombus aspiration and multiple balloon dilatations (2.0, 2.5 and 3.0 mm) at low pressure (8 and 10 atm). OCT showed complete expansion and no malapposition. Six hours after the procedure the patient developed cardiogenic shock. Immediate angiography showed a patent scaffold so no further treatment was performed. Despite optimal medical support the patient remained in cardiogenic shock and died twelve hours later at the intensive care unit (Table 5).
Discussion
In the current single-centre registry, good procedural success (95%) and acceptable midterm clinical outcomes (cumulative six-month TVF rate of 8.5%; 6.6% excluding Absorb-Tryton bifurcation case) were observed after Absorb BVS implantation in a “real-world” patient population, including high-risk patients and complex lesions. We showed that both the angiographic success rate (96%) and the QCA results are slightly lower than in ABSORB cohort B7. This small difference could be explained mainly by the composition of our population, which included more complex lesions and ACS patients.
The overall 30-day clinical outcomes of our study are worse compared to recently published data on the Absorb BVS12,13. The B-search group presented no major adverse cardiac events at 30 days in a combined patient cohort of relatively simple lesions from the ABSORB cohort A, cohort B and EXTEND, and 30-day follow-up outcomes in the BVS STEMI first study showed one case of repeat MI. The MI rate we observed was comparable with the ABSORB cohort B (3.0% vs. 3.0%), while worse outcomes were observed for cardiac death (0.8% vs. 0%), TLR (6.3% vs. 3%) and scaffold thrombosis (3.3% vs. 0%)14. These differences were mainly driven by a difference in ST rates. In addition, two TLR cases were performed because of in-stent restenosis within the Tryton stent. Excluding these patients, the six-month TLR rate was lower (5.0%), but still higher than in cohort B.
Four cases of definite ST were observed. The first case of ST could probably be explained by a distal edge dissection. The aetiology of the second case of ST was most likely underexpansion of the Absorb BVS as no pre- and post-dilatation was performed in this case. The third and fourth cases could be explained by DAPT cessation, respectively seven and five days prior to ST.
The treatment of ST was not standardised in our centre and was left to the discretion of the operator; also intracoronary imaging was not routinely performed. Limited evidence is available on the treatment of ST occurring after Absorb BVS implantation. The rationale to implant a XIENCE DES within the scaffold is debatable. In case of incomplete scaffold expansion, repeat balloon dilatations could be more suitable to treat these cases, although those could potentially result in strut fractures.
These first results may suggest that in the short term the Absorb BVS struts could potentially be more thrombogenetic compared to the cobalt-chromium struts of the XIENCE stents, due to the significantly thicker struts, 150 μm, with greater surface area which might take longer to be covered by the endothelial tissue. Although more data are needed to investigate whether Absorb is indeed more thrombogenic than contemporary DES, it is recommended to use DAPT over a period of twelve months in patients treated with Absorb BVS. Moreover, the risks of underexpansion of the scaffold may contribute to the occurrence of ST. Underexpansion of the scaffold could be avoided by adequate lesion preparation before scaffold placement, optimal sizing, and/or post-dilatation with the use of a non-compliant balloon. To limit the risk of overexpansion, the use of a non-compliant balloon is recommended, which allows post-dilatation at high pressure. Adequate lesion preparation could be achieved by predilatation using an angioplasty balloon 0.5 mm smaller than or of equal size to the scaffold. The use of cutting balloons, rotational atherectomy or thrombectomy could support lesion preparation.
In general, we found the delivery of the BVS to be more challenging in complex lesions, mainly due to the bulkier structure of the Absorb BVS compared to second-generation DES. In calcified and proximal tortuous vessels, placement of the BVS often required deeper guide intubations and more supportive wires. We also found that delivery of the Absorb BVS in distal left main coronary artery lesions is feasible, despite the inability to slowly inflate the scaffold during placement.
We believe that implantation of the Absorb BVS in a population comparable with daily clinical practice is applicable and associated with good angiographic success and acceptable midterm clinical outcomes. However, larger registries and randomised trials comparing Absorb BVS with second-generation DES with long-term follow-up are needed.
The ABSORB II randomised controlled trial, comparing the Absorb BVS with the XIENCE DES, will give valuable insights into the performance of the Absorb BVS in direct comparison with the XIENCE drug-eluting stent, particularly with regard to restoration of normal vessel vasomotion over time15. However, this trial is not powered on clinical outcomes, and inclusion is limited to stable patients with relatively simple lesions. To provide further insights into whether BVS is suitable in routine daily clinical practice the Amsterdam Investigator-initiateD Absorb strategy all-comers trial (AIDA trial) has been initiated16. This single-blinded, multicentre, randomised controlled trial will compare the Absorb BVS with XIENCE in an all-comers population.
Study limitations
This study has the intrinsic limitations of a registry. Most importantly, no control group was available to compare the use of this device with contemporary devices. QCA analyses were not performed by an independent core laboratory. Selection bias could have occurred when determining which lesions were suitable for Absorb BVS implantation. Implantation of the Absorb BVS increased as the operators gained more experience with implantations in various lesions and PCI indications. However, these procedures were performed in a highly selected patient population. The registry was confined to one centre and included 135 patients. Although clinical outcomes were obtained, no angiographic or other invasive imaging follow-up was systematically performed.
Conclusions
In a patient registry reflecting daily clinical practice, including both high-risk lesions and patients, the use of the Absorb BVS is associated with a good procedural safety and angiographic success rate, and acceptable clinical outcomes at midterm follow-up. Long-term follow-up and further evaluation in a prospective randomised controlled “all-comers” trial are needed to evaluate further the success of the Absorb BVS.
| Impact on daily practice This report suggests that implantation of the Absorb BVS in more complex patients and lesions, comparable with daily clinical practice, is feasible. However, to diminish the risk of scaffold thrombosis, it is of particular importance to prepare the lesion properly prior to BVS implantation. Moreover, DAPT should be prescribed for a period of 12 months after implantation. |
Conflict of interest statement
J.J. Wykrzykowska receives consultancy fees from Abbott Vascular and Tryton Medical. M.J. Grundeken receives consultancy fees from Tryton Medical. J.P.S. Henriques receives research grants from Abbott Vascular. J.J. Piek is a member of the Medical Advisory Board of Abbott Vascular. The AMC Heart Center received a restricted research grant from Abbott Vascular. The other authors have no conflicts of interest to declare.
Appendix. Description of patients with target lesion revascularisation
Recurrent angina in the first patient due to residual stenosis of the SB led to XIENCE Xpedition stent (Abbott Vascular) placement within the Tryton (Tryton Medical, Inc). The other patient, who also presented with recurrent angina, had progression of his residual stenosis of the SB within the Tryton which was treated with balloon dilatation using a paclitaxel-eluting balloon. The Absorb BVS (Abbott Vascular) stent in the main branch of the first patient was without any restenosis and well apposed; however, angiography and optical coherence tomography (OCT) of the second patient showed in-stent restenosis of the Absorb BVS. The patient was treated with an additional Combo sirolimus-eluting endothelial progenitor cell-capturing stent (OrbusNeich Medical, Ft. Lauderdale, FL, USA) within the scaffold. The third patient initially presented with NSTEMI and was treated in the proximal RCx. Six months after BVS implantation the patient presented with recurrent stable angina. Angiography showed a subtotal stenosis proximal to the previously placed BVS in the RCx, which was subsequently treated with an Orsiro DES (BIOTRONIK SE & Co. KG, Berlin, Germany). The last TLR case was a patient initially treated with three overlapping Absorb BVS in the LAD who had recurrent angina based on in-stent restenosis of the most distal Absorb, and who was treated by placement of a XIENCE DES (Abbott Vascular) within the scaffold.