Abstract
Aims: Bioresorbable vascular scaffolds (BVS) have been available on the European market since November 2011. The ASSURE registry aims to investigate the safety and efficacy of the Absorb everolimus-eluting bioresorbable vascular scaffold in a real-world setting.
Methods and results: Patients with de novo coronary artery disease were consecutively enrolled at six German centres in this prospective registry. Outcomes were procedural success, cardiovascular death, myocardial infarction, and ischaemia-driven target lesion revascularisation (TLR). Angiographic parameters were assessed quantitatively and visual estimates of lesion dimensions were studied. One hundred and eighty-three patients were treated. In 128 (64.7%) lesions a complex ACC/AHA morphology was present. Procedural success was achieved in all patients. Acute gain was 1.54±0.51 mm, resulting in a final minimal lumen diameter (MLD), which met the baseline reference vessel diameter (RVD), although visual estimates overrated the RVD by 0.5±0.5 mm. Up to 12 months, one patient (0.5%) had died from gastrointestinal bleeding, three (1.7%) non-target vessel myocardial infarctions occurred, and five (2.8%) TLR had become necessary because of restenosis.
Conclusions: One-year results suggest that bioresorbable vascular scaffolds for de novo coronary artery disease are associated with favourable clinical and functional outcomes in routine clinical practice despite a visually overestimated RVD.
Introduction
Bioresorbable vascular scaffolds (BVS) are transient implants. Therefore, they may be safer than metallic drug-eluting stents (DES) in terms of late stent thrombosis and neoatherosclerosis1,2. However, they consist of a lactic acid-based polymer with lower tensile properties than metal, which may impact on their procedural success regarding fracture or malapposition.
Previous ABSORB studies have proved the safety and efficacy of the BVS under clinical study conditions3,4. However, in a real-world setting including patients with a worse health status, a higher proportion of complex lesions and waiving obligatory IVUS or OCT guidance, BVS treatment has not been evaluated. For this purpose, we set up the ASSURE registry (an Absorb post-marketing surveillance registry to monitor the everolimus-eluting bioresorbable vascular scaffold in patients with coronary artery disease; ClinicalTrials.gov: NCT01583608).
Methods
PATIENTS
Consecutive patients aged between 18 and 75 years with ischaemic heart disease and one or more de novo native coronary artery lesions were enrolled between April 2012 and March 2013 at six German centres in a prospective, observational registry. Lesions with a reference vessel diameter of ≥2.0 mm and ≤3.8 mm and a percent diameter stenosis of ≥50% were eligible for BVS treatment.
STUDY DEVICE
The second-generation Absorb bioresorbable vascular scaffold (Absorb™; Abbott Vascular, Santa Clara, CA, USA) consists of a bioresorbable poly-L-lactic acid-based polymer coated with everolimus and the bioresorbable poly(D,L-lactic acid) (PDLLA)-based polymer (PDLLA) in a 1:1 ratio.
During the first six months of enrolment, only 3.0 mm BVS 18 mm in length were available. From then onwards, 2.5 mm BVS (18 and 28 mm in length), 3.0 mm BVS (28 mm in length), and 3.5 mm BVS (12, 18, and 28 mm in length) were also available and used by the ASSURE investigators. The 2.5 and 3.0 mm scaffolds were identical but crimped onto different diameter balloons. The design of the 3.5 mm scaffold incorporated a higher diameter expansion.
PROCEDURE
Patients not on chronic antiplatelet therapy received an oral loading dose of clopidogrel 600 mg and i.v. aspirin 500 mg at the time of the intervention. During the procedure, patients received appropriate anticoagulation according to standard hospital practice. Regarding the BVS, all patients were maintained on 75 mg clopidogrel daily and 100 mg aspirin for a minimum of six months. Aspirin 100 mg daily was continued thereafter.
Predilation of the target lesion was mandatory. If, however, the deployed scaffold size was still inadequate with respect to the reference vessel diameter, a high-pressure balloon was used for post-dilation. Post-dilation beyond 3.0 mm in case of a 2.5 mm BVS or beyond 3.5 mm in case of a 3.0 mm BVS or beyond 4.0 mm in case of a 3.5 BVS was avoided, because of the risk of scaffold damage.
Overlap of multiple BVS in long lesions was allowed. To avoid gap restenosis, the overlap had to be at least 1 mm and no more than 4 mm. In case of dissection requiring intervention a further BVS or DES was implanted.
ANGIOGRAPHIC ANALYSIS
Angiographic analysis was performed by the core laboratory of the University of Ulm (Ulm, Germany) using CAAS version 5.7 software (Pie Medical Imaging BV, Maastricht, The Netherlands).
The analyses comprised a qualitative analysis including lesion location, vessel morphology, lesion characteristics, blood flow, angiographic complications, and separately performed quantitative standard measures of the in-scaffold segment, the 5 mm proximal, and the 5 mm distal peri-scaffold segments. The in-lesion/in-scaffold reference vessel diameter (RVD) was determined by the virtual point of intersection of an interpolated diameter line generated from the proximal to the distal edge of the segment at the site of minimal lumen diameter (MLD). Acute gain was calculated as MLD post scaffold implantation minus MLD pre-scaffold implantation.
ENDPOINTS
Outcomes of equal weight were the composite of cardiovascular death, myocardial infarction and ischaemia-driven TLR (MACE), as well as target vessel failure (TVF), target vessel revascularisation (TVR), coronary artery bypass grafting (CABG), stroke/TIA, procedural success, functional outcome, and acute gain. The target lesion was defined as the treated segment from 5 mm proximal to the scaffold and up to 5 mm distal to the scaffold. Procedural success was defined as the achievement of <50% residual stenosis and TIMI flow 3 without the occurrence of MACE during hospital stay. Functional outcome was defined as the proportion of patients presenting angina pectoris. Bleeding was defined according to Bleeding Academic Research Consortium (BARC) definitions.
STATISTICS
Continuous variables are reported as mean±standard deviation or median (interquartile range [IQR]). Categorical variables are presented as frequencies and percentages. To identify predictors for acute gain, improvement of percentage diameter stenosis (DS) and increase of RVD from baseline to final, linear mixed models were used in order to adjust for the cluster structure induced by the different centres. All models were baseline-adjusted. Covariates at patient level, vessel level, and procedure level were tested and kept in the model if significant. Moreover, to evaluate the association of the procedural RVD increase with BVS length to a possible enhanced dilation at the edges by pressure, the two-way interaction of BVS length and implantation pressure was included in the RVD model. The results are presented as parameter estimates and their corresponding 95% confidence intervals (CI). A two-tailed p-value <0.05 was considered statistically significant. All analyses were performed using STATA 13 (StataCorp. 2013) (StataCorp LP, College Station, TX, USA).
Results
PATIENTS, LESIONS, AND PROCEDURE
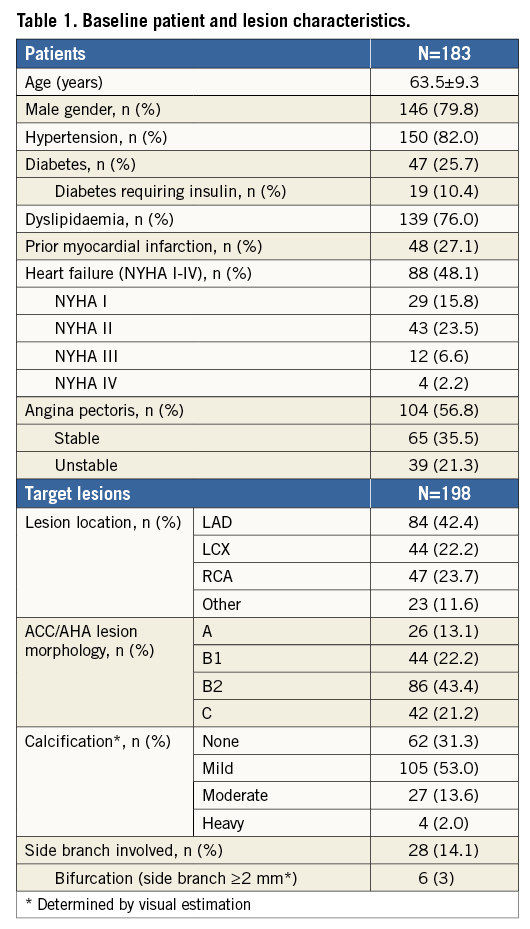
A total of 183 patients (63.5±9.3 years, 79.8% male) were enrolled and 198 lesions treated. Six-month follow-up was completed by 183 patients and 12-month follow-up by 180. The prevalence of diabetes, hypertension and dyslipidaemia was 25.7%, 82.0% and 76%, respectively.
Median lesion length was 11.6 mm (IQR 9.3 to 16.5 mm) with 37 (18.7%) lesions being >20 mm. A complex ACC/AHA lesion morphology of B2 or C was seen in 128 (64.7%) lesions. Thirty-one (15.7%) lesions were moderately or heavily calcified, six (3%) lesions were tightly angulated, and six (3%) lesions involved a side branch of ≥2 mm in diameter. The reference vessel diameter of 88 (44.4%) lesions was ≤2.5 mm (Table 1, Table 2).
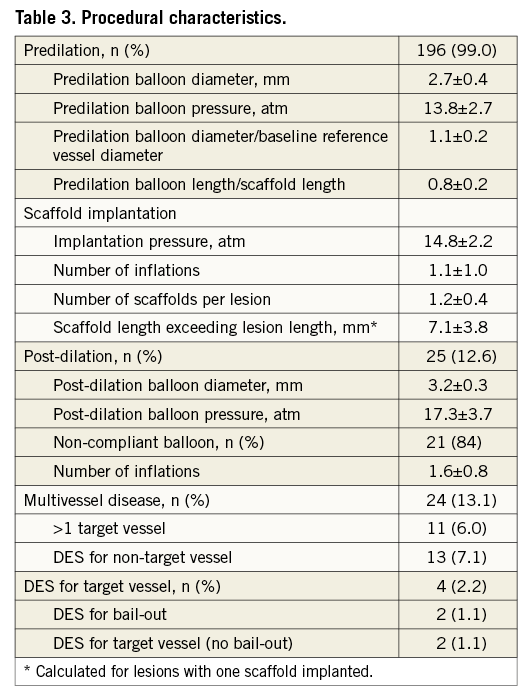
Predilation was performed in 196 (99%) lesions with a balloon to vessel diameter ratio of 1.1±0.2 and a balloon to scaffold length ratio of 0.8±0.2. For scaffold implantation, a median pressure of 16 atm (IQR 14-16 atm) was used. Scaffolds exceeded the lesion length by 7.1±3.8 mm. Post-dilation was performed in 25 (12.6%) lesions with a median pressure of 18 atm (IQR 15-20 atm) with 1.6±0.8 inflations per lesion, mainly (84%) by using a non-compliant balloon (Table 3). In 30 (15.2%) lesions >1 BVS was implanted, along with BVS overlap in 18 cases.
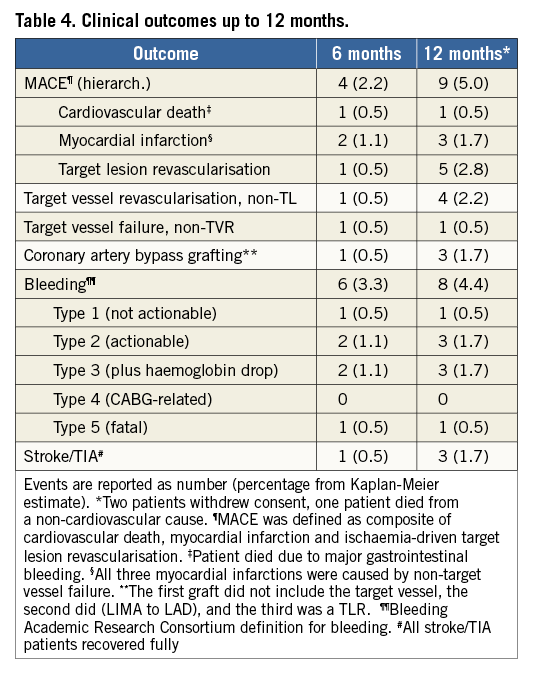
CLINICAL OUTCOME
Acute procedural success, defined as <50% residual stenosis and TIMI 3 flow without occurrence of MACE during hospital stay, was achieved in all patients. No dissection was reported from the core laboratory. However, in two eccentric lesions a DES bail-out was conducted.
At 12 months, one (0.5%) death due to gastrointestinal bleeding under dual antiplatelet therapy and three (1.6%) myocardial infarctions, caused by non-target vessel failure, had occurred.
TLR had to be carried out in five patients (2.8%). Two of them with long lesions (38.4 mm and 24.0 mm) of small vessels (RVD 1.7 mm and 2.3 mm), treated with overlapping 3.0 mm BVS, were revascularised at six and seven months by paclitaxel-coated balloon. A third patient had an in-scaffold restenosis of a vein graft to the proximal right circumflex artery (CX) due to BVS malapposition, confirmed by IVUS at seven months, with a distal BVS deformation from balloon re-angioplasty, necessitating a drug-eluting stent implantation. In a fourth patient TLR was performed due to an incomplete proximal BVS expansion in the CX, noticed by OCT at eight months, resolved by conventional balloon angioplasty. The fifth patient with a left main/LAD lesion needed CABG treatment because of a total occlusion of the proximal LAD at 11 months.
Another in-scaffold restenosis of a long proximal LAD lesion (26.6 mm, RVD 2.6 mm) of 50% diameter at six weeks after the index procedure did not require revascularisation.
Post procedure one patient suffered from angina due to a reduced flow (TIMI 2), which resolved during immediate re-angiography without further intervention.
Target vessel revascularisation was required in four patients. One patient had to be treated with DES proximally and distally to the mid CX target lesion at six and 12 months. A second patient had to be treated with DCB and BVS distally to the mid LAD target lesion at nine months, a third patient needed a LIMA graft to the LAD at nine months, and a fourth patient had to be treated for target vessel stenosis distally to the mid LAD target lesion with another slightly overlapping BVS at 12 months (Table 4).
At six and 12 months, angina pectoris was markedly less frequent and severe (Figure 1).
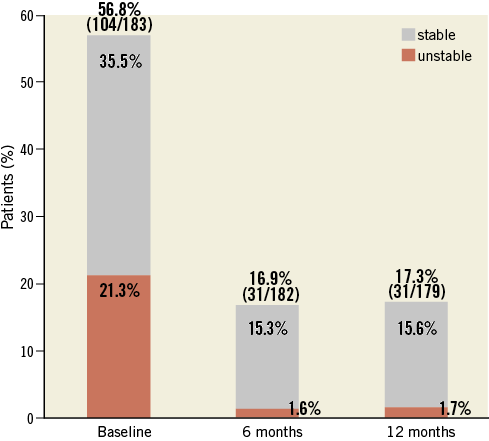
Figure 1. Angina pectoris at baseline, six, and 12 months.
Of 31 (17.3%) patients presenting with angina pectoris at 12 months, nine were revascularised at target or non-target lesions.
LESION LUMINA
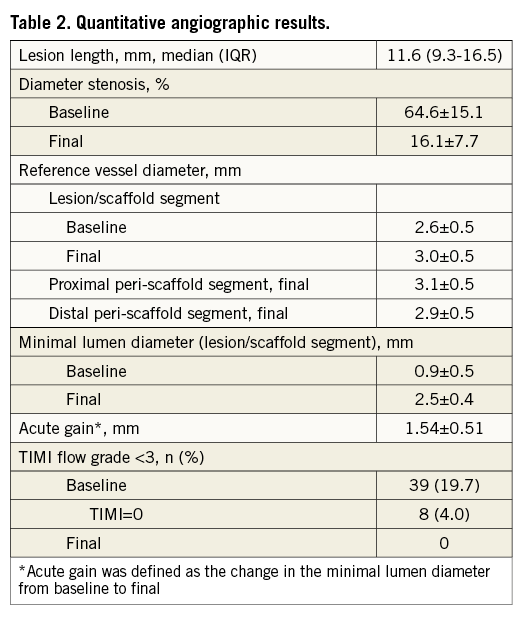
Diameter stenosis was 64.6±15.1% at baseline, with eight (4%) total occlusions, and improved to a residual diameter stenosis of 16.1±7.7% (Table 2). The improvement of diameter stenosis in long lesions (>20 mm) was less by 4.1% (95% CI: 0.77-7.4, p=0.016) as compared to shorter lesions. Centre-specific mean age and proportion of diabetes were associated with less improvement of diameter stenosis (1.5% worse per year of mean centre’s patient’s age [p=0.011] and 1.2% worse per 1% diabetics per centre [p=0.001], respectively).
Acute gain of MLD was 1.54±0.51 mm. Centre-specific mean baseline patient characteristics, such as gender, diabetes and dyslipidaemia had an effect on the acute gain (p<0.001). None of the lesion- or procedure-related variables showed a statistically significant association with the acute gain.
The RVD increased after BVS by 0.3±0.4 mm from baseline to final, thereby aligning with the level of the peri-scaffold segments. In case of 28 mm BVS, the implantation pressure increased the RVD by 0.06 mm per atmosphere (95% CI: 0.01-0.11, p=0.031).
Acute recoil from the maximal lumen to the post-procedural lumen was greater at the site of MLD than at the lesion edges (0.49±0.45 mm vs. 0.02±0.49 mm, respectively).
Compared to QCA, visual estimation overrated the baseline RVD by 0.5±0.5 mm and the DS by 13.2±16.5%. Nevertheless, the final MLD closely matched the target of baseline RVD (Figure 2A). In case of a BVS-RVD mismatch and use of a 3.0 mm BVS the target MLD was achieved for small vessels (<2.5 mm), but not for large vessels (>3.3 mm). A BVS-RVD mismatch resulted in an optimal MLD but an overexpanded RVD in case of 3.0 mm BVS for <2.5 mm vessels, whereas in 3.3 mm vessels final MLD fell below the RVD (Figure 2B). Final RVD exceeded the BVS expansion limit in 20 (10.1%) lesions, accompanied by a higher implantation pressure (15.4±1.3 atm) and a higher frequency of post-dilation (20.0%, 4/20 lesions). Lesions treated with a 2.5 mm BVS were more frequently affected in this respect (final RVD >3 mm in 10/30 lesions, 33.3%) compared to lesions treated with a 3.0 mm BVS (final RVD >3.5 mm in 9/107 lesions, 8.4%) or 3.5 mm BVS (final RVD >4.0 mm in 1/61 lesions, 1.6%) (Figure 2C).

Figure 2. Lesion diameters and BVS sizing. A) Visual overestimate of RVD with final MLD closely matching baseline RVD. B) BVS-RVD mismatch for 3.0 mm BVS. C) Final RVD exceeding BVS >0.5 mm.
Discussion
MACE
The 12-month MACE incidence of 5.0% in ASSURE was in the same range as in previous BVS studies and in everolimus-eluting metallic stent (EES) trials, even though ASSURE MACE included a fatal gastrointestinal bleeding event. The MACE rate in ABSORB Cohort B (BVS) was 6.9%5, in ABSORB EXTEND 4.3%6, and in SPIRIT IV (EES) 4.1%7. The three non-fatal myocardial infarctions in ASSURE were not related to the target lesion. No scaffold thrombosis occurred (Figure 3). The high rate of patients still receiving dual antiplatelet therapy at 12 months (75.6%) may possibly have prevented scaffold thrombosis.
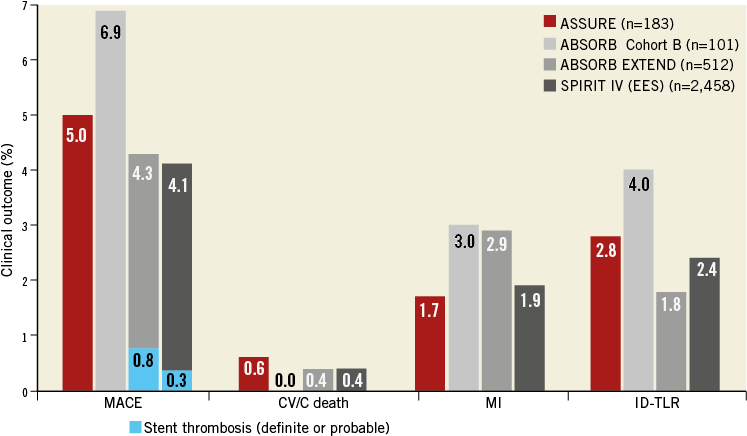
Figure 3. MACE in ASSURE and in the ABSORB Cohort B, ABSORB EXTEND, and SPIRIT IV trials at 12 months. CV/C death: cardiovascular/cardiac death; EES: everolimus-eluting stent; ID-TLR: ischaemia-driven target lesion revascularisation; MACE: major adverse cardiovascular events (defined as CV/C death, MI or ID-TLR)
Two TLRs involved long lesions (>20 mm) in small vessels (≤2.5 mm), in which the RVD was visually overestimated. A higher acute gain of 0.7±0.2 mm in small vessels compared to 0.5±0.2 mm in vessels >2.5 mm, also reported by Diletti et al5, as well as BVS overlap might have contributed to both restenoses. The TLR rate for small-vessel procedures with BVS overlap was 22.2%. The three other TLRs were possibly related to lesion complexity as they involved a vein graft, a left main/LAD bifurcation, and an eccentric proximal CX stenosis.
Real-world experience
THE CHALLENGE OF COMPLEXITY
Not only the health status of patients in terms of baseline cardiovascular diseases but also the complexity of the lesions were worse in the ASSURE registry than in ABSORB Cohort A, ABSORB Cohort B and EXTEND patients, as evaluated in the B-SEARCH study8 (AHA/ACC lesion morphology B2 or C: 64.6% vs. 32.6%). Lesions were longer and percentage DS was greater in ASSURE lesions compared to interim reported ABSORB EXTEND9 lesions (15.0±11.0 mm vs. 11.7±4.9 mm; 64.6±15.1% vs. 58.6±10.6%, respectively). Also, the amount of calcification (68.7%), side branch involvement (14.1%) and total occlusions (4%) in the ASSURE registry represented real-world conditions. Nevertheless, in ASSURE the acute gain of 1.54 mm was higher than known from previous ABSORB clinical studies with 1.10 to 1.25 mm on average8,10. Therefore, the final MLD got closer to the target of baseline RVD. Acute gain from everolimus-eluting metallic stents has been reported as similar to11-13 or greater than7 that from BVS in ASSURE (Figure 4). Percentage DS improved similarly with BVS and EES (ASSURE 64.6% to 16.1% vs. B-SEARCH 60.5% to 16.9% vs. SPIRIT II 61% to 13%). Post-dilation was performed less frequently in the ASSURE registry (12.6%) than in the B-SEARCH study (55%) and in the SPIRIT IV trial (45.1%), but the maximum pressure in ASSURE (15.2±2.3 atm) was similar to the maximum pressure applied for EES in SPIRIT IV (15.3±2.9 atm) and exceeded the maximum pressure in ABSORB studies (14.1±2.8 atm)8. Additionally, slightly higher pressures for predilation (13.8 atm vs. 12.6 atm) as well as a higher predilation balloon/RVD ratio (1.1 vs. 0.9) might have contributed to an increased acute gain in the ASSURE registry compared to previous ABSORB studies.
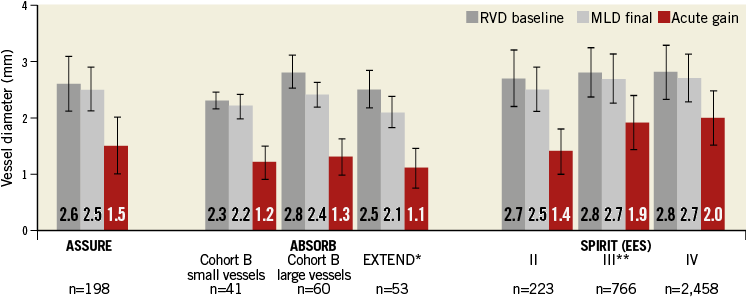
Figure 4. Comparison of lesion diameters between ASSURE and clinical BVS trials (ABSORB Cohort B, ABSORB EXTEND) and EES trials
(SPIRIT II, III, and IV). *ABSORB EXTEND lesions from B-SEARCH; **Stone GW for the SPIRIT III Investigators, unpublished, New Orleans 2007
ERROR OF VISUAL ESTIMATION (Figure 2A)
Compared to DES, BVS sizing may be more important in terms of malapposition and fracture because of the lower tensile strength of the lactic acid polymer14,15. Baseline RVDs and percentage DS were visually overestimated compared to the QCA-determined values, as has been previously reported for small vessels by Diletti et al10. As a consequence, investigators chose BVS of larger sizes and used higher pressures more often. Possibly as a result of this unintentionally ambitious approach, the acute gain was higher and the final MLD got closer to the target of RVD as compared to the IVUS-supported ABSORB Cohort A and B studies.
There was no significant correlation between implantation pressure and acute gain. Only for long BVS (28 mm) was the implantation pressure linearly related to an RVD increase from baseline to final. As RVD was indirectly determined from the diameters at the lesion edges, long BVS may possibly have dilated lesion edges more than 18 mm BVS did.
In 150 (75.8%) lesions investigators determined proximal and distal Dmax16 by QCA before BVS implantation. Baseline RVD by visual estimate (3.1±0.4 mm) matched the mean Dmax (3.0±0.3 mm) well. The difference of 0.2±0.3 mm between proximal (3.0±0.4 mm) and distal lesion edges (2.9±0.4 mm) was small. Therefore, Dmax did not provide added value to time-saving visual estimates in our experience.
MATTER OF MISMATCH (Figure 2B)
Large vessels (RVD >3.3 mm) treated with 3.0 mm BVS finally achieved an MLD of 0.5±0.5 mm less than baseline RVD, even though scaffolds were implanted with relatively high pressure (16.0±1.2 atm). Despite favourable results in terms of acute gain (1.66±0.38 mm) as well as an improvement of diameter stenosis from 59.9% to 14.4%, the use of 3.5 mm BVS might have been beneficial.
Satisfactory angiographic results for small vessels <2.5 mm treated with 3.0 mm BVS confirmed the findings of Diletti et al10 who described similar percentage residual stenosis and late lumen loss for small vessels compared to vessels ≥2.5 mm treated with 3.0 mm BVS at six months. However, Diletti et al did not report any difference in the clinical outcome and a similar restenosis rate in patients with small vessels treated with 3.0 mm BVS. However, in the ASSURE registry two of the five ischaemia-driven TLR were performed in patients with long lesions in small vessels, visually overestimated in terms of RVD and dilated with balloons exceeding the QCA-RVD. In case of long lesions with a small RVD, a more cautious treatment in terms of diameter and pressure could perhaps be appropriate to avoid exaggerated vessel wall irritation.
LIMIT OF EXPANSION (Figure 2C)
Although the 2.5 and 3.0 mm scaffolds are the same, the crimping process limits their expansion. The 2.5 mm scaffold can be taken up to 3.0 mm, the 3.0 mm scaffold to 3.5 mm and the 3.5 mm scaffold to 4.0 mm. However, the latter has a slightly different design to allow for this amount of expansion. In our patients, a final RVD which exceeded the expansion limit of the BVS was reached in 20 (10.1%) lesions. A BVS diameter of 2.5 mm and a comparably high implantation pressure or post-dilation may have contributed to this outcome.
Limitations
During the first six months of the registry only 3.0 mm BVS 18 mm in length were available, leading to mismatches of lesion and scaffold size. The ASSURE registry was a non-randomised observational study with a limited number of patients.
Conclusions
At 12 months, BVS for de novo coronary artery disease were safe and effective in a real-world setting with a relatively high amount of complex lesions and without obligatory IVUS or OCT. The frequency of MACE concurs with previous BVS and DES studies5,7. Angina pectoris improved substantially. Acute gain and residual diameter stenosis were similar to everolimus-eluting metallic stents. Visual overestimation of baseline RVD and DS compared to QCA resulted in higher predilation and implantation pressures and a higher mean predilation-balloon/RVD ratio than known from IVUS-guided BVS studies8,11.
| Impact on daily practice BVS implantation without IVUS or OCT guidance appears to result in acute angiographic and 12-month clinical outcomes similar to DES. A high BVS expansion pressure and slight BVS oversizing seem to be the key factors to achieve these goals. Angina pectoris symptoms can be expected to be markedly relieved after BVS. In this registry, no patient experienced a stent thrombosis within a 12-month follow-up period. The overall one-year MACE rate was 5% and thus comparable to previous DES and BVS studies. Unlike metallic stents, BVS are limited by their expansion capacity. To achieve an optimal angiographic result, it is therefore important to choose the correct BVS size carefully. If this is done, BVS seem to be safe and effective and may become a useful alternative to DES in many patients undergoing coronary stent implantation. |
Acknowledgements
The authors thank Richard Rapoza, PhD, for his valuable advice.
Funding
This work was supported by an unrestricted research grant from Abbott Vascular, Deutschland GmbH.
Conflict of interest statement
The authors have no conflicts of interest to declare.

