
Abstract
Aims: The aim of this study was to analyse the procedural results and midterm safety of everolimus-eluting bioresorbable vascular scaffolds (BVS) used for percutaneous coronary intervention in a large all-comers cohort from the German-Austrian ABSORB RegIstRy (GABI-R).
Methods and results: A total of 3,231 patients were included in this prospective, observational, multicentre study (ClinicalTrials.gov NCT02066623) of consecutive patients undergoing BVS implantation between November 2013 and January 2016. Endpoints were major adverse cardiac events (MACE; a composite endpoint of death, target vessel revascularisation, and myocardial infarction), and target lesion failure (TLF; a composite endpoint of cardiac death, target vessel myocardial infarction, and target lesion revascularisation). Scaffold thrombosis was a further endpoint. Of all patients, 51.5% presented with acute coronary syndrome. Predilatation and post-dilatation were performed in 91.5% and 71.9% of patients, respectively. Procedural success was 98.9%. After six months, the incidence of MACE was 4.1% and of TLF 2.4%. The rate of target vessel MI was 1.5%, and target lesion revascularisation was performed in 1.8%. Definite/probable scaffold thrombosis was documented in 1.4% of patients.
Conclusions: GABI-R, the largest registry to provide data regarding safety after BVS implantation in a real-world setting, reveals high procedural success and low six-month event rates.
Abbreviations
ARC: Academic Research Consortium
BVS: bioresorbable vascular scaffold(s)
DES: drug-eluting stent
MACE: major adverse cardiac events
MI: myocardial infarction
TLF: target lesion failure
TLR: target lesion revascularisation
TVF: target vessel failure
TVR: target vessel revascularisation
Introduction
Several randomised controlled trials have demonstrated that poly-L-lactic acid-based everolimus-eluting bioresorbable vascular scaffolds (BVS; Abbott Vascular, Santa Clara, CA, USA) appear to be similar in terms of safety and effectiveness compared to current-generation metallic drug-eluting stents (DES) for the percutaneous treatment of coronary artery stenosis, despite a higher risk of scaffold thrombosis1. Two recent trials have shown significantly more events during long-term follow-up2,3. However, most randomised studies share strict inclusion criteria, and consequently a large proportion of patients are underrepresented or even excluded. Nevertheless, it is essential to verify the results of these trials in a real-world setting.
The retrospective GHOST-EU registry, which is the largest all-comers registry on BVS to date, also demonstrated unexpectedly high event rates4. However, it has been shown that lesion morphology and implantation techniques may influence clinical outcomes5,6.
The present interim analysis of the prospective German-Austrian ABSORB RegIstRy (GABI-R) provides detailed procedural results and midterm safety outcomes of the largest prospective cohort of patients treated with BVS in daily clinical practice.
Methods
STUDY DESIGN AND PATIENT COHORT
GABI-R is a prospective, observational, multicentre study (ClinicalTrials.gov NCT02066623) of consecutive patients undergoing BVS implantation at 92 sites in Germany and Austria between November 2013 and January 2016. This investigation was approved by the local ethics boards. All patients received and signed a written consent. Data sets were collected centrally via an electronic case report form provided by the IHF (Institut für Herzinfarktforschung, Ludwigshafen, Germany); thus, source verification and quality control were conducted independently. Monitoring was performed once in 30 participating clinics, which included at least 10 patients each. The informed consent was checked in every case and all source data were verified in five patients. Follow-up via questionnaire or telephone interview was predefined by protocol after 30 days, six months, two and five years. Additional details have been published previously7.
PROCEDURAL DETAILS
The implantation technique complied with the current standards and, albeit not mandatory, predilatation and post-dilatation were strongly recommended8. The use of intravascular imaging was left to the implanting physician’s discretion. High-pressure balloon dilatation was defined as dilatation at ≥14 atm. Procedural success was defined as a visually estimated residual stenosis <30% within the treated segment. Dual antiplatelet therapy was prescribed for at least one year in all patients.
TARGET PARAMETERS
The primary endpoints were major adverse cardiac events (MACE), including cardiac death, target vessel revascularisation (TVR), or myocardial infarction (MI), and target lesion failure (TLF), including cardiac death, clinically driven target lesion revascularisation (TLR), or target vessel MI. The composite endpoint of target vessel failure (TVF) comprises cardiac death, target vessel MI, and clinically driven target vessel revascularisation. Clinically driven TLR was defined as ≥50% diameter stenosis and recurrent angina, objective signs of ischaemia, abnormal results of invasive functional testing or ≥70% diameter stenosis even in the absence of the previously noted criteria9. Scaffold thrombosis, according to the Academic Research Consortium (ARC) criteria, was also evaluated9. Cardiac death was defined as death from an immediate cardiac cause or complications related to the procedure or any death in which a cardiac cause could not be excluded. MI was defined according to the World Health Organization extended definition10.
All events were adjudicated by an independent events committee based on clinical charts and review of the angiograms. There was no quantitative core laboratory involved for adjudication. The events committee was not involved in the study.
STATISTICAL ANALYSIS
Qualitative data were analysed as absolute values and percentages, and continuous variables are presented as means with standard deviation. Time-to-event data were visualised using Kaplan-Meier (product-limit) estimates. P-values for the homogeneity of time-to-event (survival) curves were calculated by the log-rank test. A small number of missing event dates were completed by random hot-deck imputation (one imputed date used in this analysis). In addition, a comparison was made between patients treated in 2014 and patients treated in 2015 and later. Continuous data were compared with the Wilcoxon rank test, categorical data with the chi-square test and Kaplan-Meier estimates with the log-rank test.
Results
BASELINE CHARACTERISTICS
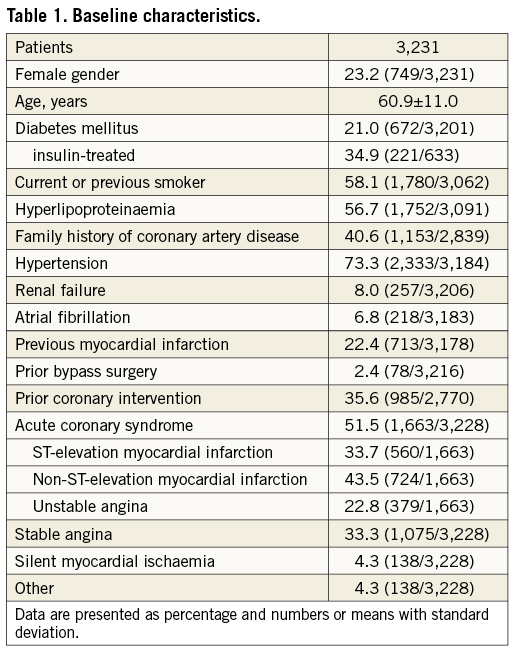
A total of 3,231 patients with a mean age of 60.9±11.0 years and 23.2% being female were included. Diabetes was present in 21.0%, hypertension was noted in 73.3% and hyperlipidaemia in 56.7%. An acute coronary syndrome at hospital admission was present in 51.5%. Further baseline characteristics are presented in Table 1.
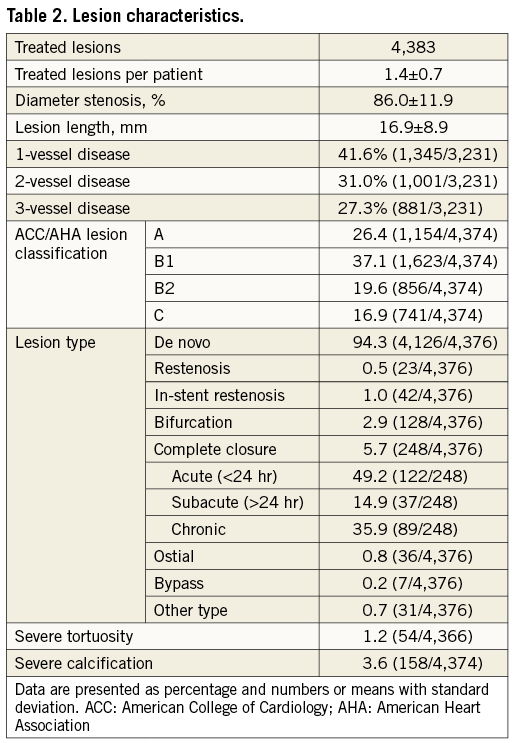
A total of 4,383 lesions were treated. Mean visually estimated lesion length was 17.1±9.2 mm, and 6.7% of patients had lesions >34 mm. Of all lesions, 36.5% were classified B2/C according to the ACC/AHA classification and bifurcated lesions were present in 2.9%. Further lesion-related characteristics can be found in Table 2.
PROCEDURAL RESULTS
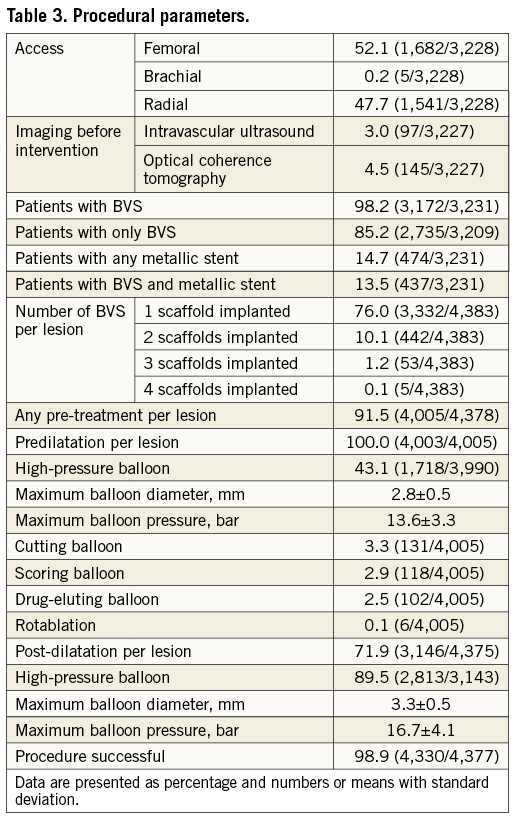
Pre-treatment was performed in 91.5% of all lesions, including high-pressure predilatation at ≥14 atm in 43.1%. Overall, 98.2% of the patients were treated with BVS. In 86.4% of the treated lesions, BVS were used alone, in 10.7% metallic stents were used alone, and in 2.9% BVS and DES were used in a “hybrid approach”. The mean diameter of the implanted devices was 3.1±0.6 mm and the mean length was 19.7±6.2 mm. An overlap was performed in 13.8% of patients. Additional post-dilatation was applied in 71.9% of the lesions, of which 89.5% were performed at high pressure (≥14 atm). Procedural success was achieved in 98.9%.
Of all patients discharged, 97.3% were on aspirin, 97.6% on an ADP receptor antagonist (clopidogrel 44.2%, prasugrel 34.0% and ticagrelor 34.0%), and 8.0% on oral anticoagulation. Further procedural details are provided in Table 3.
SIX-MONTH OUTCOME
Six-month follow-up was completed in 99.3% of all patients and the median follow-up duration was 204 (195-230) days. At the six-month follow-up, 93.2% were on aspirin, 93.1% on an additional ADP receptor antagonist (clopidogrel 44.1%, prasugrel 33.2%, and ticagrelor 22.7%), and 8.5% on oral anticoagulation. The incidences of MACE, TLF and TVF were 4.1%, 2.4% and 3.6%, respectively. The TLR rate was 1.8%, the TVR rate was 3.2%, and the ARC-defined definite scaffold thrombosis rate was 1.0%. Of the 31 definite scaffold thromboses, eight occurred on the same day as the index procedure. An overview of the six-month results is displayed in Table 4 and Kaplan-Meier curves are shown in Figure 1. There were no statistically relevant differences found regarding patients in whom predilatation and post-dilatation was performed in all lesions compared to all other patients. When comparing patients undergoing implantation according to a retrospectively defined implantation protocol (maximum predilatation balloon and maximum BVS diameter ratio of 1:1 and additional post-dilatation with a balloon size of the BVS diameter up to 0.5 mm above in all lesions), there were also no relevant differences found (Table 5).
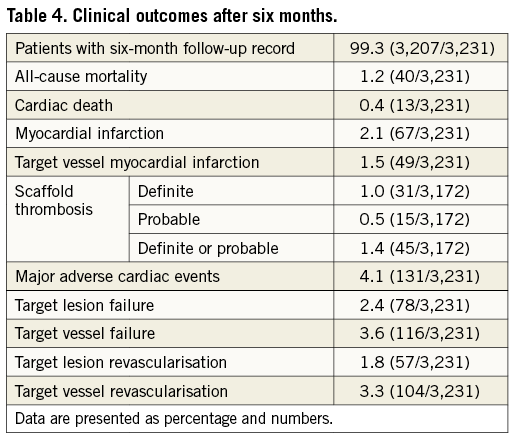
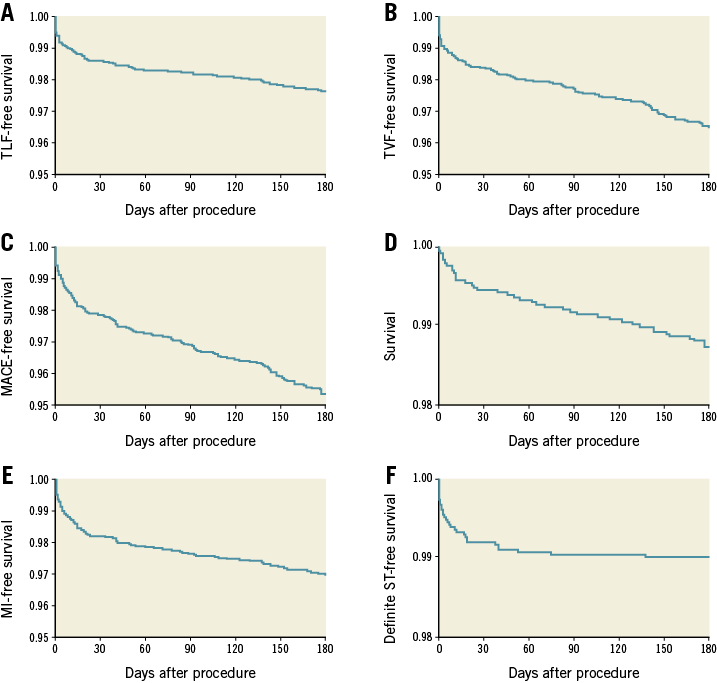
Figure 1. Six-month outcomes of the GABI-R population. A) Kaplan-Meier curves of freedom from target lesion failure (cardiac death, clinically driven target lesion revascularisation or target vessel myocardial infarction). B) Freedom from target vessel failure (cardiac death, target vessel MI, or clinically driven target vessel revascularisation). C) Freedom from major adverse cardiac events (death, target vessel revascularisation, or myocardial infarction). D) Survival. E) Freedom from myocardial infarction. F) Freedom from definite scaffold thrombosis.
COMPARISON OF PATIENTS TREATED BEFORE AND AFTER 1 JANUARY 2015
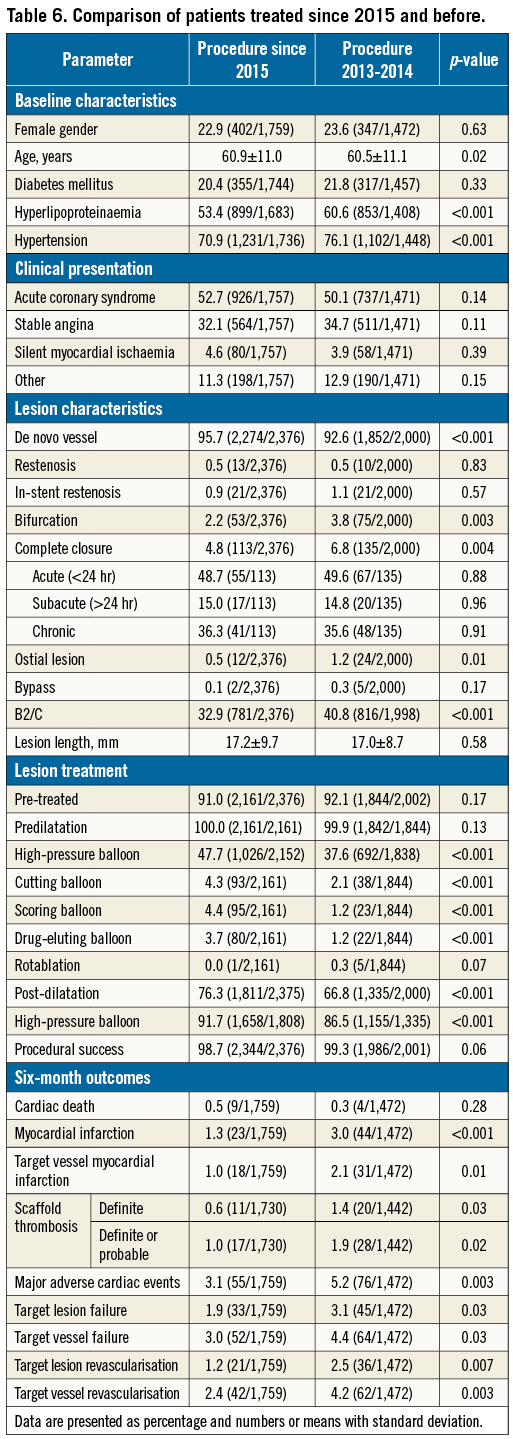
A total of 1,759 patients were treated after 1 January 2015; 1,472 had undergone BVS implantation in 2014 or earlier. There were no protocol changes regarding patient selection or implantation technique within the enrolment period. However, a change of the implantation technique can be observed since the proportion of patients with predilatation and post-dilatation in all treated lesions increased from 58.6% to 67.4% (p<0.001), as well as the proportion of patients who underwent BVS implantation according to a retrospectively defined implantation protocol from 22.2% to 29.9% (p<0.001). When comparing patient characteristics, a slightly less severe cardiovascular risk profile was observed in the group of patients treated in 2015 and later. The clinical presentation was similar in both groups, but patients treated in 2015 and later had less complex lesions. Furthermore, more thorough lesion preparation was performed in 2015 and later. Also, post-dilatation was more often applied with a greater use of high-pressure balloons. Significantly lower six-month event rates occurred during the follow-up of patients treated in 2015 and later (Figure 2). Further details are presented in Table 6.
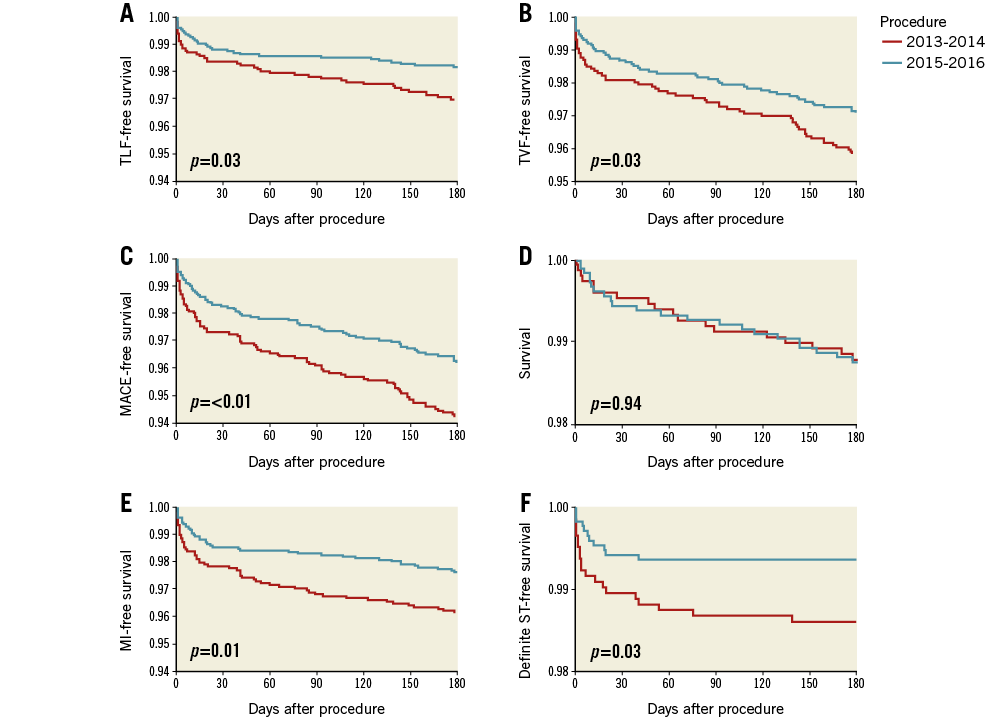
Figure 2. Comparison of outcomes of patients treated in 2015 versus earlier. A) Kaplan-Meier curves comparing freedom from target lesion failure (cardiac death, clinically driven target lesion revascularisation or target vessel myocardial infarction). B) Freedom from target vessel failure (cardiac death, target vessel MI, or clinically driven target vessel revascularisation). C) Freedom from major adverse cardiac events (death, target vessel revascularisation, or myocardial infarction). D) Survival. E) Freedom from myocardial infarction. F) Freedom from definite scaffold thrombosis.
Discussion
The present study shows that BVS implantation in daily clinical practice in a heterogeneous patient cohort is associated with high technical success. Midterm outcomes, including six-month follow-up results, demonstrate clinical safety. Finally, there was a tendency for less complex lesions and a more thorough predilatation and post-dilatation among patients treated in 2015 versus earlier, resulting in significantly lower six-month event rates.
There are currently several randomised controlled trials comparing BVS and DES. In ABSORB III, a total of 2,008 patients were treated with either BVS or an everolimus-eluting DES (XIENCE; Abbott Vascular)11. After one year, the TLR rate was 3.0% and 2.5% (p=0.50) in the BVS and XIENCE groups, respectively, whereas scaffold/stent thrombosis occurred in 1.5% and 0.7% (p=0.13)12. In addition, the AIDA trial evaluated 1,845 patients who were randomised 1:1 to DES. After two years no differences regarding composite endpoints were noted, but a significantly higher definite scaffold thrombosis rate was observed for BVS (3.1% vs. 0.6%; p<0.001)3.
Most of these trials had strict inclusion criteria as patients with acute coronary syndromes were excluded as well as those with lesions ≥24 mm or ostial and bifurcated lesions. Thus, various settings are underrepresented, which reflects the need for all-comers studies without restrictions.
The GHOST-EU registry includes almost 1,200 patients treated with BVS in daily clinical routine. The six-month TLR and scaffold thrombosis rates were 2.5% and 2.1%, respectively4. Notably, the median interval from implantation to scaffold thrombosis was 6.5 days; thus, procedural and non-device-related factors were probably the cause, underscoring the need for optimisation of the implantation technique. The GABI-R RegIstRy demonstrated a lower six-month event rate for TLF (2.4% vs. 4.4%) and a lower rate for definite/probable scaffold thrombosis (1.4% vs. 2.1%) than those observed in GHOST-EU4. Furthermore, a significant decrease of event rates during 2015 compared to the first two years of GABI-R has been observed. However, the overall scaffold thrombosis rate is still higher than for current DES11,12.
Following a learning curve, clinical outcome after BVS implantation may improve6, and a refined implantation technique has been shown to reduce event rates13. Additionally, an analysis of the GHOST-EU registry demonstrated that systematic predilatation, adequate sizing and systematic post-dilatation (“PSP”) are associated with improved outcome5. An improvement in the implantation technique has also been observed in GABI-R: for example, the post-dilatation rates per lesion in GHOST-EU, GABI-R 2013-2014 and GABI-R 2015 were 49%, 67% and 76%, respectively. The analysis of patients treated in 2015 vs. those treated earlier also showed that fewer patients with complex lesions underwent BVS implantation. In comparison to GHOST-EU, the proportion of bifurcated lesions, aorto-ostial lesions, and cases of total occlusion and restenosis in GABI-R is substantially lower. It is known that BVS implantation in these complex lesions yields higher event rates14,15.
Limitations
The enrolment was not performed in a randomised fashion. A total of 3,231 patients were included during the protocol-mandated enrolment period, which is less than originally planned. The reasons for that are speculative, but most probably data from several randomised trials published during the enrolment period raised concerns regarding the adverse event rates, especially scaffold thrombosis. In addition, more information on patient and lesion selection was available which further limited the indication for BVS, which is also reflected in the change of lesion and patient characteristics through the course of the enrolment period of this registry. Reimbursement issues in Germany may also have had an influence. Since the discretion to implant a BVS was the only relevant inclusion criterion and left to the physician, BVS use consequently decreased, which led to a premature termination of the registry. Cardiac enzymes were not routinely assessed post PCI in every participating centre and thus under-reporting of periprocedural MI may be possible. Patients who underwent concomitant DES implantation were also included, which may have influenced interpretation of the data. There were no protocol-mandated criteria predefined regarding the selection of either a BVS or a metallic DES and the decision was left to the implanting physician’s discretion. One further limitation of this investigation is that there was no specific sizing modality required to support optimal BVS selection. In almost all cases (96.4%), a visual estimation of the vessel size was performed, which cannot be as precise as online QCA measurements or even intravascular imaging techniques. Routine angiographic follow-up was not scheduled for patients in this registry, and the analysis does not include any angiographic outcome measurements. The midterm results presented here constitute an interim analysis, and patient follow-up will continue in order to obtain long-term data.
Conclusions
This analysis of initial results of GABI-R, the largest cohort of unselected patients treated with BVS in clinical routine to date, demonstrates high procedural success and low event rates. However, there is a trend towards a higher rate of scaffold thrombosis as compared with DES registries at six months. A tendency towards treatment of patients with less complex lesions and use of a more dedicated implantation technique during the later phase of the inclusion period was associated with a reduction in event rates. Long-term data regarding safety endpoints as well as quality of life and economic factors will be required to test the proof of concept on different levels; these data will also be provided in time by GABI-R.
| Impact on daily practice GABI-R is the largest reported patient cohort evaluating BVS implantation in daily routine. Midterm results reveal low event rates, supporting the finding that BVS implantation during daily practice is safe. Within the inclusion period, a trend towards less complex lesions and more dedicated implantation techniques was observed, resulting in lower event rates. This may help to identify optimal patients for BVS implantation. |
Acknowledgements
The authors thank Elizabeth Martinson, PhD, for her editorial assistance, and Dr Steffen Schneider from the Institut für Herzinfarktforschung, Ludwigshafen, Germany, and Dipl.-Ing. Thomas Pfannebecker, Abbott Vascular, for their support and input.
Funding
The GABI Registry is supported by Abbott Vascular, Santa Clara, CA, USA.
Conflict of interest statement
H. Nef reports receiving research grants (institutional) and speaker honoraria from Abbott Vascular. G. Richardt is a member of the advisory board of Abbott Vascular. S. Achenbach reports receiving research grants (institutional) from Abbott Vascular and Siemens Healthcare. J. Mehilli has received lecture fees from and is on the advisory board of Abbott Vascular and Terumo, also lecture fees from Lilly/Daiichi Sankyo and Boston Scientific, and an institutional research grant from Abbott Vascular and Edwards Lifesciences. C. Hamm has received speaker honorarium from Medtronic and consulting fees from Abbott Vascular and Medtronic. The other authors have no conflicts of interest to declare.

