
Abstract
Aims: The aim of this study was to assess clinical restenosis and its predictors after implantation of bioresorbable vascular scaffolds (BVS) in everyday practice in the large-scale German-Austrian ABSORB Registry (GABI-R).
Methods and results: Between November 2013 and January 2016, 3,264 patients underwent BVS implantation in the 93 centres of GABI-R (ClinicalTrials.gov NCT02066623). At six-month follow-up, 24 patients experienced clinically indicated target lesion revascularisation (cTLR) unrelated to BVS thrombosis (cumulative incidence 0.76%; angiographically, 58.3% of in-BVS restenosis of focal pattern). Compared to patients without cTLR, patients with cTLR had more lesions per patient (1.83±1.0 vs. 1.36±0.7), complex (52.3% vs. 36.2%) and mild-to-moderately calcified lesions (65.9% vs. 60.5%) treated, and more frequently had overlapping BVS (22.2% vs. 10.8%), all p<0.05. Implanted BVS length was 40.0 mm (28.0, 46.9) vs. 23.0 mm (18.0, 30.0), p<0.001, remaining in the multivariable analysis the only independent predictor of cTLR (hazard ratio 1.02, 95% CI: 1.01-1.04, p<0.001). The myocardial infarction rate was also significantly higher among patients with cTLR, 29.2% vs. 1.7%, p<0.0001.
Conclusions: cTLR related to BVS restenosis at six months after BVS implantation is a rare event depending on implanted BVS length. Whether cTLR increases the myocardial infarction risk needs to be evaluated at longer-term follow-up and within the setting of adequately powered randomised trials.
Abbreviations
BVS: bioresorbable vascular scaffold
CI: confidence interval
cTLR: clinically indicated target lesion revascularisation
DES: drug-eluting stents
HR: hazard ratio
MI: myocardial infarction
PCI: percutaneous coronary intervention
SD: standard deviation
Introduction
Restenosis with the need for repeat revascularisation, although infrequent, continues to represent a clinical risk during long-term follow-up after implantation of drug-eluting stents (DES)1. Therefore, stent technologies, which allow temporary scaffolding of the vessel while providing a device-free coronary tree at longer term, are an attractive concept. Of the currently available fully bioresorbable drug-eluting platforms, the Absorb™ (Abbott Vascular, Santa Clara, CA, USA) scaffold – a polylactic acid-based bioresorbable vascular scaffold (BVS) is the most frequently used and the most extensively clinically tested device. After the first studies reported very promising data at short- and long-term follow-up, a series of randomised studies was started2-7. Available results indicate a risk of scaffold thrombosis which is higher than for DES, and which seems to be strongly dependent on lesion selection and implantation technique8-10. On the other hand, the reported incidences of clinically indicated target lesion revascularisation (cTLR) were comparabale between BVS and modern everolimus-eluting stents3,6. Several anatomical and procedural factors such as calcified and ostial lesions, small vessel size, lack of adequate lesion preparation or BVS overlapping have been identified as increasing this risk8,11. These findings have led to a more rigorous selection of lesions considered for BVS implantation and to the use of a strictly standardised implantation procedure. To monitor the clinical usage and performance of BVS in everyday practice, the German-Austrian ABSORB Registry (GABI-R) was designed – a prospective registry enrolling consecutive patients undergoing BVS implantation at 93 centres in two countries12. The large number of prospectively included patients and detailed characterisation of the population in this registry allow the assessment of adverse events and their predictive factors. In the current analysis we aimed to assess the incidence of clinical restenosis and its predictors within six months after BVS implantation.
Methods
Between November 2013 and January 2016, 3,264 patients underwent implantation of the latest version of the BVS (Absorb BVS) in the 93 GABI-R centres. As published previously12, GABI-R is an observational study and patient participation in this registry has no impact on further management. BVS use was left to the operators’ discretion. The recommended BVS implantation procedure followed most recent standards and has been been published previously13. The extent of lesion preparation and use of intracoronary imaging (for BVS sizing reasons) was left to the individual operator’s discretion. Antiplatelet therapy consisted of aspirin and a P2Y12 receptor inhibitor for at least 12 months.
The study was conducted in accordance with the provisions of the Declaration of Helsinki. The ethics committees of the participating institutions approved the registry protocol. All patients provided written, informed consent.
DATA MANAGEMENT, DEFINITIONS AND OUTCOME OF INTEREST
Data within GABI-R are collected electronically via an internet-based application and centralised at the Institut für Herzinfarktforschung (IHF GmbH, Ludwigshafen, Germany). All events were adjudicated and classified by an independent events adjudication committee.
The incidence of clinical restenosis and identification of its predictors are the outcomes of interest of the current analysis. Clinical restenosis was defined as clinically indicated percutaneous or surgical TLR not related to BVS thrombosis. Scaffold thrombosis was defined as definite or probable according to Academic Research Consortium (ARC) criteria. Cardiac death was defined as death from immediate cardiac cause or complications related to the procedure as well as any death in which a cardiac cause could not be excluded. Myocardial infarction (MI) was defined according to the World Health Organization extended definition3. Target lesion failure (TLF) was defined as a composite of cardiac death, target vessel MI, and cTLR. Target vessel failure (TVF) was defined as a composite of cardiac death, target vessel MI, and clinically indicated target vessel revascularisation (TVR).
STATISTICAL ANALYSIS
Distributions of metric variables within the two groups, with and without cTLR, are described by means±standard deviations or median (quartiles). Binary variables are described by absolute frequencies and percentages. Frequencies of outcomes are complemented by odds ratios and their 95% confidence limits, where possible. All descriptive statistics are based purely on known (non-missing) values. To explain the incidence of in-BVS restenosis by baseline and procedural variables, a multiple proportional hazards (Cox) regression model was built. Missing values were imputed either by random drawing from the standardised empirical distribution (in case of missing times-to-event), by modal values (binary) or by median values (metrical variables). Predictor variables in the model were pre-specified initially and reduced after assessing the results of a preliminary model. A two-tailed p-value <0.05 was considered to indicate statistical significance. Statistical analysis was performed using SAS software, version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
PATIENT AND PROCEDURAL CHARACTERISTICS
At six-month follow-up, of the 3,264 BVS-treated patients enrolled, 86 patients (2.6%) had unknown vital status (mean follow-up 49±2 days) and 32 patients (1.0%) had unknown clinical status. Of the total cohort of 3,146 patients eligible for the current analysis, 24 patients (cumulative incidence 0.76%) (Figure 1) experienced cTLR. Both patient groups - with or without cTLR - were comparable regarding their baseline profile (Table 1).
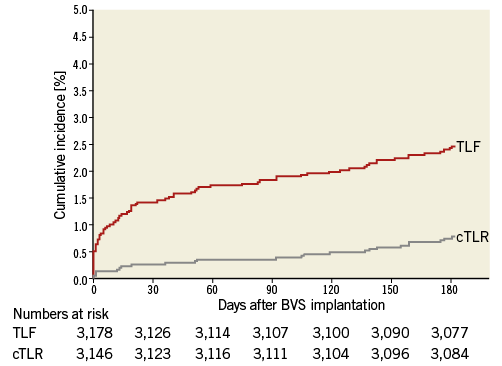
Figure 1. Clinically indicated target lesion revascularisation and target lesion failure at six-month follow-up after BVS implantation. BVS: bioresorbable vascular scaffold; cTLR: clinically indicated target lesion revascularisation; TLF: target lesion failure
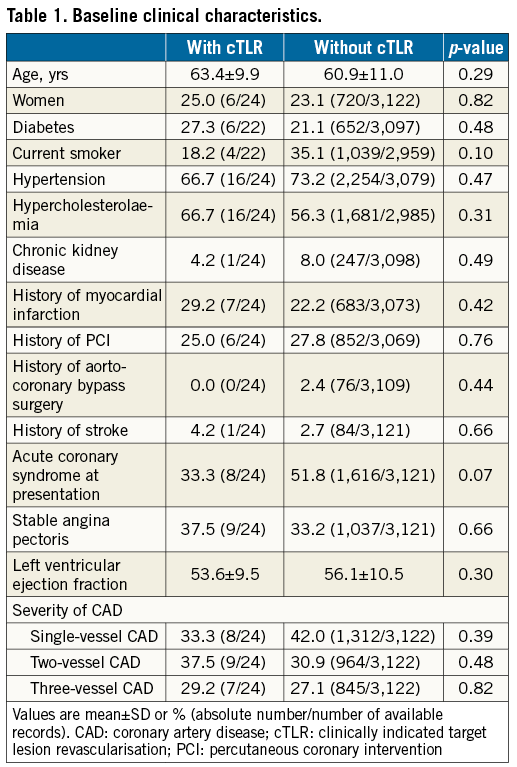
Compared to patients without cTLR, patients with cTLR underwent more complex procedures requiring significantly longer radiation times, had a higher number of treated lesions/patient, and more complex and severely calcified lesions. In addition, they more frequently had overlapping and longer implanted BVS (Table 2). No differences in chronic antiplatelet therapy at admission and at discharge were observed, while a significantly higher proportion of patients with cTLR was on angiotensin receptor blocker and nitrate therapy at six-month follow-up (Table 3).
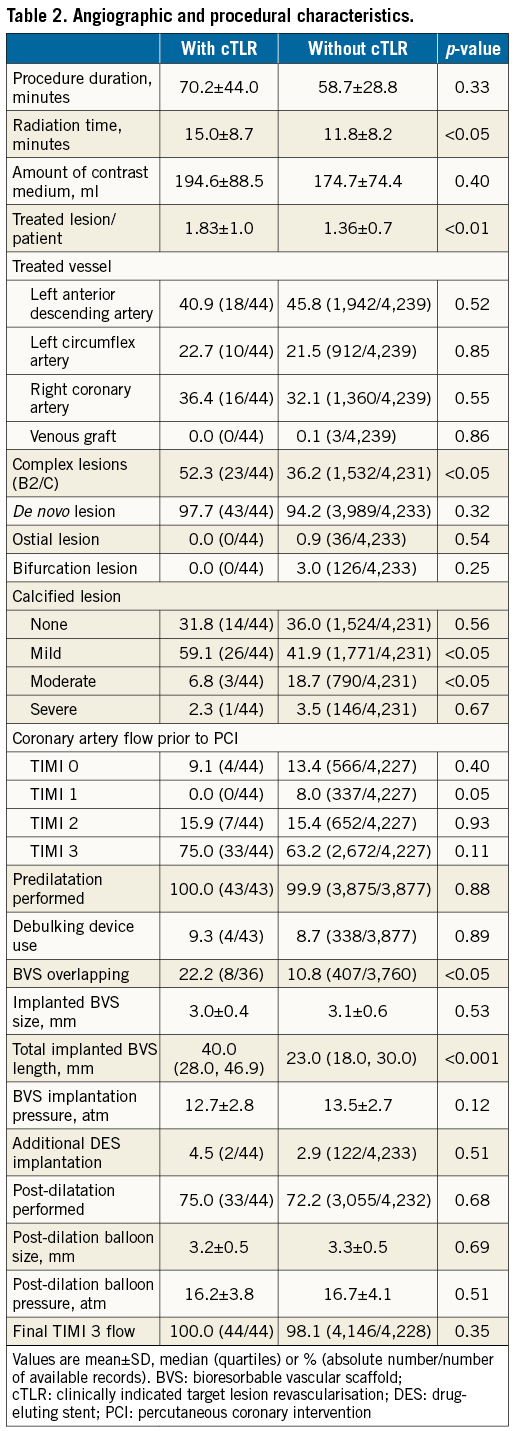
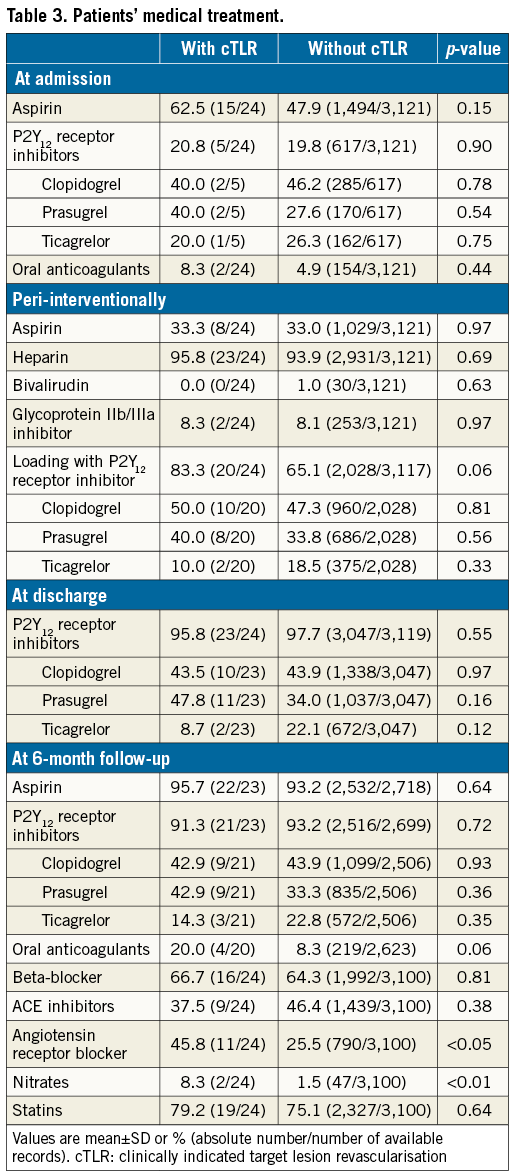
CLINICAL OUTCOMES
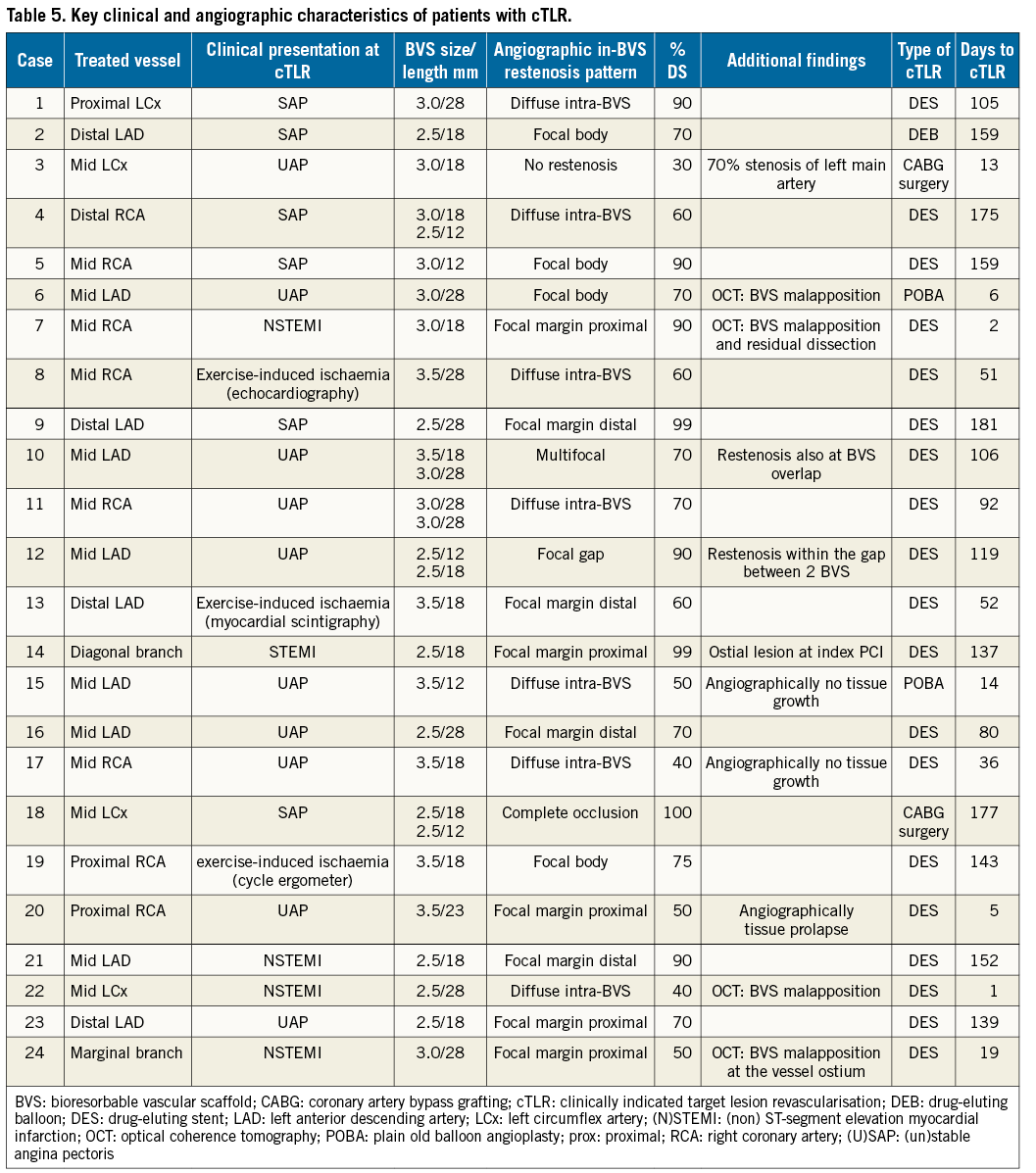
The mean follow-up period was 212±21 days among cTLR patients, and 219±36 days in patients without cTLR, p=0.98. A total of 38 patients died, all in the substantially larger group of patients without cTLR. Definite scaffold thrombosis occurred in 30 of the 3,122 patients without cTLR (1.0%) and one patient (0.4%) in the cTLR group after re-PCI for BVS restenosis (Table 4). The overall incidence of MI during follow-up was significantly higher among patients with cTLR (29.0% vs. 1.7%, p<0.001). Angiographically, the majority of in-BVS restenosis had a focal pattern, while malapposition was observed among cases with early in-BVS restenosis undergoing optical coherence tomography (Table 5).
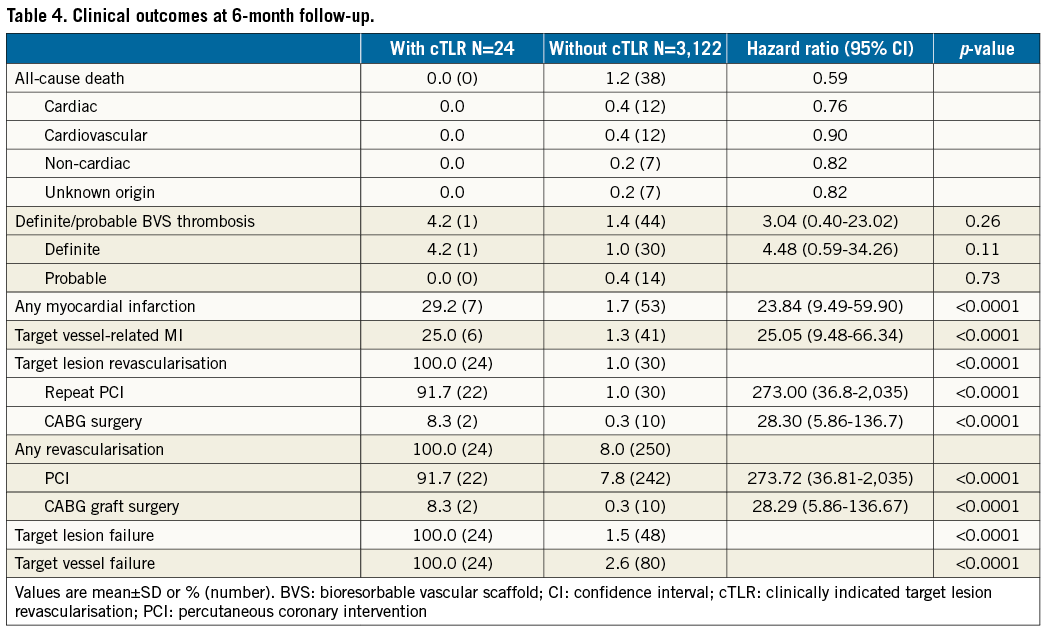
For multivariable analysis, next to the factors that significantly differed between the two groups, traditional predictors of restenosis were forced into the model. Except total implanted BVS length (per each mm increase, hazard ratio [HR] 1.02, 95% CI: 1.01-1.04, p<0.001), none of the other factors (calcified lesion, HR 0.41 [0.12-1.41], p=0.16; complex lesion, HR 0.90 [0.38-2.12], p=0.81; implanted BVS size, HR 0.79 [0.23-2.73], p=0.71; diabetes, HR 1.48 [0.58-3.78], p=0.41) independently predicted the risk of cTLR after BVS implantation.
Discussion
GABI-R is the largest investigator-initiated international registry monitoring routine use and outcomes after BVS implantation. In the current analysis of more than 3,000 patients, we found that: i) the incidence of cTLR related to in-BVS restenosis was extremely low at six months after BVS implantation; ii) none of the traditional factors except implanted BVS length independently predicted the risk of clinical in-BVS restenosis; and iii) the majority of angiographic in-BVS restenoses have a focal pattern and in-BVS restenoses occurring early after implantation are frequently caused by BVS malapposition.
The ongoing process of restenosis in the very long term after DES implantation and the need for treatment of recalcitrant restenosis created the scientific basis for development of BVS platforms1,14. The polylactic acid-based Absorb BVS, the first commercially available BVS being used on a routine basis, has been evaluated in an extensive programme of registries and randomised trials. While the overall performance of the scaffold was found to be good, several trials and meta-analyses have revealed a slightly, but significantly increased risk of scaffold thrombosis as compared to thrombosis of metal-based DES3-7,9,10. On the other hand, the necessity for revascularisation due to restenosis seemed to be comparable among BVS and DES3,6. In the Comparison of Everolimus- and Biolimus-Eluting Stent with Everolimus-Eluting Bioresorbable Scaffold Stents (EVERBIO) II trial, the BVS platform was randomly compared with two DES platforms regarding the amount of angiographic late lumen loss at nine-month angiographic follow-up. The incidence of TLR was 10% after BVS implantation and 8% after DES implantation, p=0.59. These findings are supported by the recently published Amsterdam Investigator-initiateD Absorb strategy all-comers (AIDA) randomised trial which was powered for clinical endpoints (n=1,845). The trial required no routine angiographic follow-up and the incidence of cTLR due to restenosis was 2.4% with Absorb and 2.5% with DES at one-year follow-up6. Compared to randomised trials, much lower rates of TLR unrelated to thrombosis have been reported for real-world cohorts. In the GHOST-EU registry, of the 1,189 enrolled patients, 25 patients required in-BVS revascularisation (cumulative incidence, 2.5%) due to thrombosis (n=20) or restenosis (n=5) at six-month follow-up4. In the current analysis of GABI-R, we also observed a very low rate of cTLR (0.76%). Lack of routine angiographic follow-up, patient selection and particularly lesion selection play an important role in explaining the very low rate of this complication. In our registry, 23% of lesions were of complex morphology. The use of BVS in bifurcation lesions and ostial lesions was discouraged and occurred in only 3% of cases. This is a relevant difference in comparison to the AIDA trial, where 55% of BVS-treated lesions were of complex and 10% of ostial or bifurcational morphology6. On the other hand, widespread use of appropriate lesion preparation and BVS implantation techniques as encouraged in GABI-R (predilatation in all cases and post-dilatation in approximately ¾ of all patients) might have contributed to the low cTLR15.
Diabetes mellitus, vessel size (device size), stented length, residual stenosis or stent overlap have frequently been identified as predictors of restenosis after percutaneous coronary intervention16. In the current study, only the total length of the implanted BVS was identified as a predictor of cTLR at follow-up. The very selected nature of the population and lesions in the GABI-R, technically more demanding procedures among patients with cTLR leading to additional BVS implantation and BVS size-lesion mismatch might have played a role in these findings. Another explanation might be the type of in-BVS restenosis. Nearly 25% of cTLR occurred within the first 30 days and were therefore most likely not related to neointimal hyperplasia (intracoronary imaging of some of these cases supports this). The lower radial strength with polylactic acid-based BVS as compared to DES might lead to early restenosis within BVS due to elastic recoil17.
Generally, restenosis is considered a benign event being either asymptomatic or with stable clinical presentation. However, in the GABI-R, about one quarter of patients with cTLR presented with target vessel MI compared to only 1.3% of patients without cTLR (1% due to BVS thrombosis). Long-term follow-up of large registries and randomised trials will be required to corroborate our observations and to investigate further the mechanisms and consequences of in-BVS restenosis.
Limitations
GABI-R is a prospective clinical registry, which inherently harbours patient selection bias, heterogeneity regarding lesion preparation and PCI technique, as well as a certain degree of incompleteness of data collection. On the other hand, determination of restenosis or thrombosis at the time of revascularisation was based on centrally reviewed angiograms. However, lack of systematic intracoronary imaging at the time of cTLR might have hampered the evaluation of the true incidence and aetiology of in-BVS restenosis. Finally, a follow-up duration of six months is probably not sufficient to evaluate the clinical performance of BVS. GABI-R will follow all patients for up to five years after BVS implantation, which will allow a detailed time-dependent characterisation of restenosis after BVS implantation.
Conclusions
Clinical restenosis at six months after BVS implantation, a rare event, is associated with a greater length of implanted BVS. Whether this is related to a higher risk of myocardial infarction or other hard endpoints needs to be evaluated at longer-term follow-up and within the setting of adequately powered randomised trials.
| Impact on daily practice The rate of thrombosis after bioresorbable vascular scaffold implantation is higher than expected, but this may be addressed by meticulous lesion selection and implantation technique. Clinical restenosis is a parameter that characterises treatment success independently from scaffold thrombosis but has been infrequently reported. We demonstrate that clinical restenosis within six months after BVS implantation is an infrequent event and depends on the implanted BVS length. This is encouraging, since it highlights that implantation strategies which achieve low rates of thrombosis will not be offset by high rates of other clinical events. Furthermore, in-BVS restenosis occurring earlier after BVS implantation suggests elastic recoil as one of its possible mechanisms and emphasises that BVS implantation within vessel segments at high mechanical stress should be avoided. |
Funding
The GABI Registry is supported by Abbott Vascular, Santa Clara, CA, USA.
Conflict of interest statement
J. Mehilli has received lecture fees from and is on the advisory board of Abbott Vascular, Biotronik and Terumo, has received lecture fees from Lilly/Daiichi Sankyo and Boston Scientific, and institutional research grants from Abbott Vascular and Edwards Lifesciences. S. Achenbach has received research grants (institutional) from Abbott Vascular and Siemens Healthcare. H. Nef has received research grants (institutional) and speaker honoraria from Abbott Vascular. G. Richardt is on the advisory board for Abbott Vascular. T. Gori has received lecture fees from Abbott Vascular. A. Schmermund has received a speaker’s honorarium from Abbott Vascular. C. Hamm has received a speaker’s honorarium from Abbott Vascular. The other authors have no conflicts of interest to declare.

