
Abstract
Aims: The aim of this study was to assess outcomes following Absorb bioresorbable scaffold (BVS) implantation in an unrestricted clinical practice according to an “on-label” versus “off-label” indication.
Methods and results: RAI is a prospective registry, investigating BVS performance in different lesion subsets. No specific exclusion criteria were applied. Co-primary endpoints were target lesion revascularisation (TLR) and definite/probable scaffold thrombosis (ScT) at one year. A total of 1,505 patients (1,969 lesions) were enrolled. In 58% of patients, BVS was implanted in at least one off-label subset according to the manufacturer’s instructions for use. Predilatation was performed in 98.5% of the cases, and post-dilatation in 96.8%. At one-year follow-up, TLR and ScT rates were 3.3% and 1.3%, respectively. TLR was significantly higher in the off-label group (4.0% vs. 2.2%, HR 1.8, 95% CI: 1.0-3.4; p=0.05) while a trend towards a higher ScT rate was observed in the off-label group (1.7% vs. 0.6%, HR 2.7, 95% CI: 0.9-8.2; p=0.06). At multivariate analysis, treatment of in-stent restenosis, chronic total occlusion and BVS diameter were independent predictors of TLR.
Conclusions: Our data from a real-world population suggest that BVS could be associated with acceptable one-year clinical outcomes when meticulously implanted. However, a higher rate of adverse events was observed when this device was used in off-label lesions. (ClinicalTrials.gov: NCT02298413)
Abbreviations
ACS: acute coronary syndrome
BRS: bioresorbable scaffolds
BVS: bioresorbable vascular scaffold(s)
CTO: chronic total occlusions
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
DOCE: device-oriented composite endpoint
EES: everolimus-eluting stent
ISR: in-stent restenosis
QCA: quantitative coronary angiography
ScT: scaffold thrombosis
STEMI: ST-segment elevation myocardial infarction
TLR: target lesion revascularisation
TV-MI: target vessel myocardial infarction
TVR: target vessel revascularisation
Introduction
New-generation drug-eluting stents (DES) represent the current gold standard treatment for all subsets of patients and lesions1. Bioresorbable scaffolds (BRS) were introduced as a technological advance to eliminate the permanent caging of the coronary wall by permanent DES with the aim of restoring the pristine native vessel state2. Data comparing an everolimus-eluting BRS (Absorb™ bioresorbable vascular scaffold [BVS]; Abbott Vascular, Santa Clara, CA, USA) versus a permanent everolimus-eluting stent (EES) (XIENCE [X-EES]; Abbott Vascular) have indicated similar efficacy as well as an increased rate of target vessel myocardial infarction (TV-MI) and scaffold thrombosis (ScT) at one-year follow-up3,4. More recently, long-term reports of randomised trials have raised doubts on the “real” non-inferiority of the BVS when compared to the best in class X-EES5,6. Factors related to the device, the operator and the patient/lesion have been advocated as potential causes to explain those results.
With respect to the implantation technique, retrospective analyses have demonstrated a reduction in BVS failures when a specific deployment “protocol” was applied7,8. In this context, we investigated the one-year clinical outcomes from a large registry of real-world patients treated with BVS implantation using a low threshold for lesion predilatation and device post-dilatation.
Methods
The rationale and design of the Registro Absorb Italiano (RAI) have been described elsewhere9. The RAI registry is an independent, prospective, multicentre, Italian data collection on consecutive patients/lesions who have been successfully treated with one or more BVS between October 2012 and December 2015.
The registry reflects “real-world” BVS use, without specific exclusion criteria, allowing the treatment of complex subsets such as in-stent restenosis (ISR), thrombus-containing lesions, bifurcations, chronic total occlusions (CTO), long lesions, saphenous vein grafts (SVG), and unprotected left main (ULM) disease.
Although implantation technique was not pre-specified and left to the operator’s decision, an extensive lesion preparation with appropriately sized (1:1 balloon-to-artery ratio) semi-compliant and/or non-compliant balloons was strongly recommended, as well as BVS post-dilatation (with a maximum non-compliant balloon diameter 0.5 mm larger than the BVS nominal diameter). BVS sizing method (by visual estimation, online quantitative coronary angiography [QCA], or intravascular imaging) was left to the operator’s discretion. The following QCA parameters were assessed using different commercially available software (on-site assessment without core lab analysis): minimum lumen diameter (MLD), reference vessel diameter (RVD) obtained by an interpolated method, and percent diameter stenosis10. All patients provided informed consent for both the procedure and subsequent data collection. Dual antiplatelet therapy (DAPT) was recommended for at least six to 12 months. Patient visits were recommended at 30 days, six months, and yearly up to five years after BVS implantation. All available clinical and procedural as well as follow-up data were entered into a web-based case report form.
ENDPOINTS
Co-primary endpoints were target lesion revascularisation (TLR) and definite/probable ScT, defined according to the Academic Research Consortium criteria, at one-year follow-up11. Additionally, all patients were followed up for the incidence of a device-oriented composite endpoint (DOCE) comprising cardiac death, ischaemia-driven TLR (ID-TLR) and TV-MI.
An independent clinical events committee was constituted to adjudicate all endpoint-related events.
STATISTICAL ANALYSIS
Data are expressed as mean±standard deviation, and categorical data as numbers and percentages. An off-label BVS use was considered (according to the manufacturer’s instructions for use [IFU]) if the implantation fulfilled at least one of the following criteria: 1) RVD <2.5 mm or >3.75 mm; 2) lesion length >74 mm; 3) arterial or SVG lesion; 4) ULM lesion; 5) ostial lesion; 6) bifurcation lesion with side branch diameter >2 mm; 7) ISR; 8) severe calcification; 9) CTO; 10) three-vessel disease treated with BVS; 11) excessive tortuosity proximal to or within the lesion; and 12) ST-elevation myocardial infarction (STEMI). Comparisons of clinical, angiographic, or procedural characteristics were performed by means of the Student’s t-test or Wilcoxon rank-sum test (continuous variables), or χ² test (categorical), and on the basis of the distribution according to the lesion characteristics (on-label or off-label). Patients who were lost to follow-up were censored at their last known contact. Cumulative event rates were analysed using the Kaplan-Meier method, and the rate differences among the groups estimated using the log-rank test. Cox regression analysis was used to calculate hazard ratio (HR) and 95% confidence intervals (CI) considering the on-label group as reference.
The prognostic relevance of different variables regarding the prediction of TLR was estimated using univariable Cox regression analysis. To test the independency of variables found to be associated with an increased risk of TLR in univariable analysis, those variables with a p<0.05 were tested in the multivariable Cox proportional hazards model. All analyses were performed using SPSS software, Version 21.0 (IBM Corp., Armonk, NY, USA) and all reported p-values are two-sided. The p-values were considered significant if <0.05.
Results
During the study period, a total of 1,505 patients with 1,969 lesions were enrolled. Baseline clinical characteristics are reported in Table 1. In 873 (58%) patients (n=1,161 lesions, 59%), BVS was implanted in at least one off-label subset. Patient age was 59±10.4 years, while the overall incidence of acute coronary syndromes (ACS) was 59%, including 21% of STEMI patients.
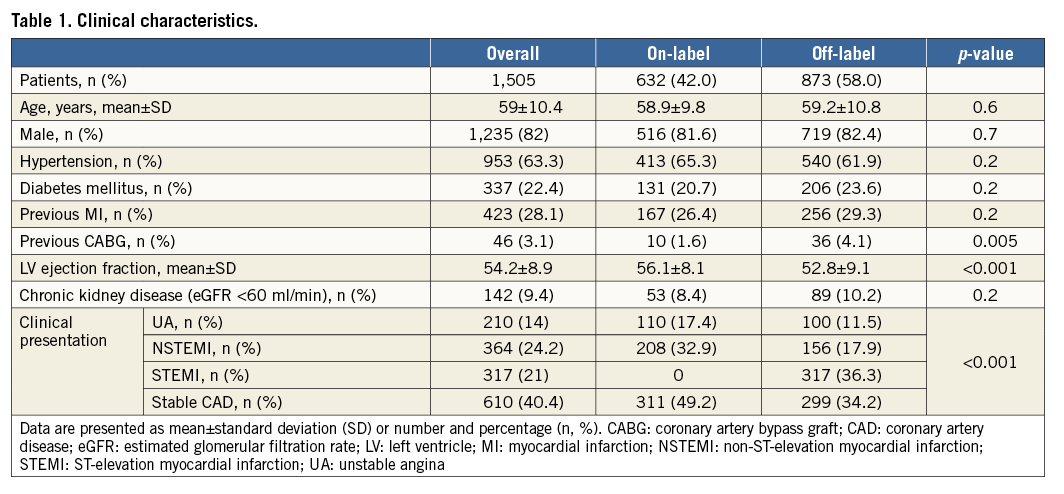
Table 2 and Table 3 show lesion/procedural and angiographic characteristics. Overall, 27.5% of the lesions were long and required BVS overlapping, 21% had moderate-to-severe calcification, 12% were bifurcations, 6.7% were ISR, and 2.9% were CTOs. Predilatation and BVS post-dilatation were performed in the vast majority of cases, and all post-dilatations were performed at high pressures (≥16 atm). Based on the pre-PCI QCA data and the latest edited IFU (version EL2109155 Rev A), an appropriate BVS sizing was performed in 72% of the cases.
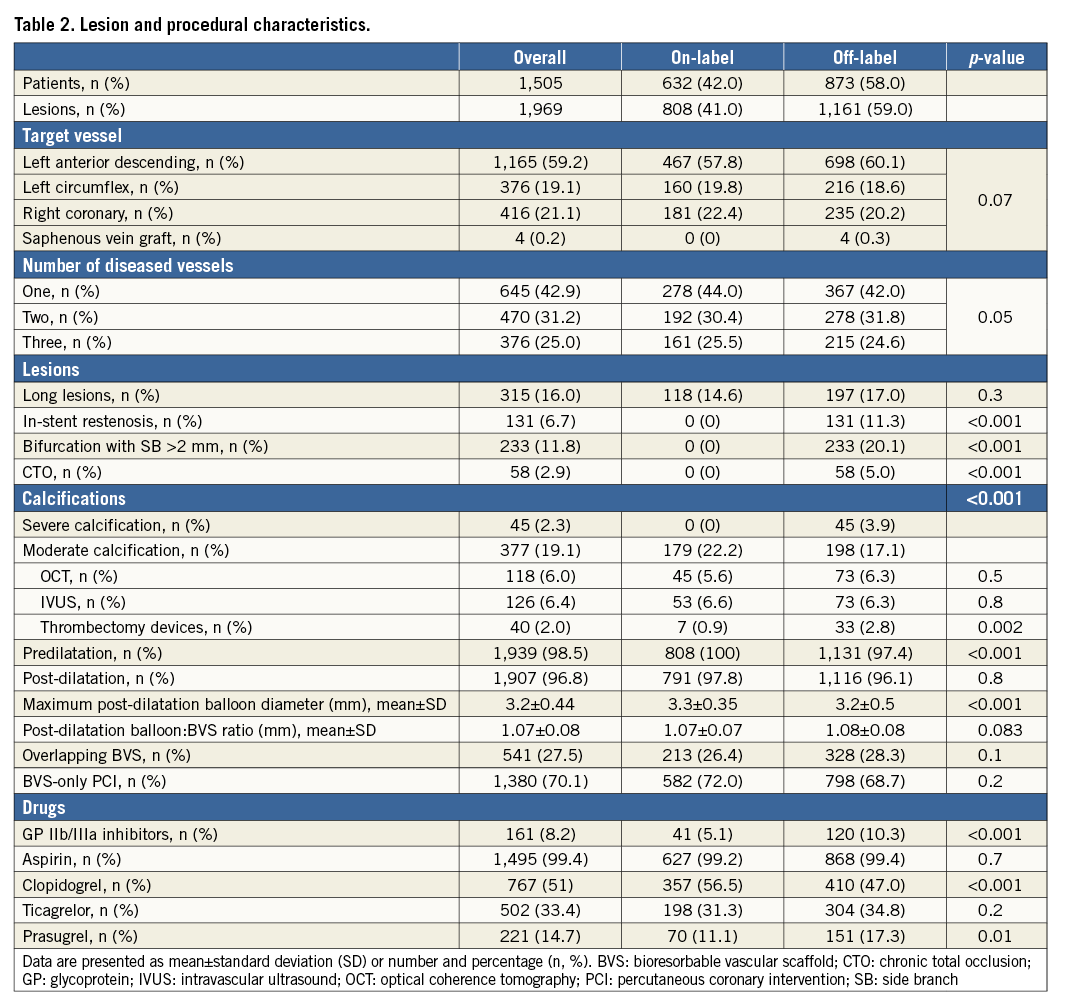
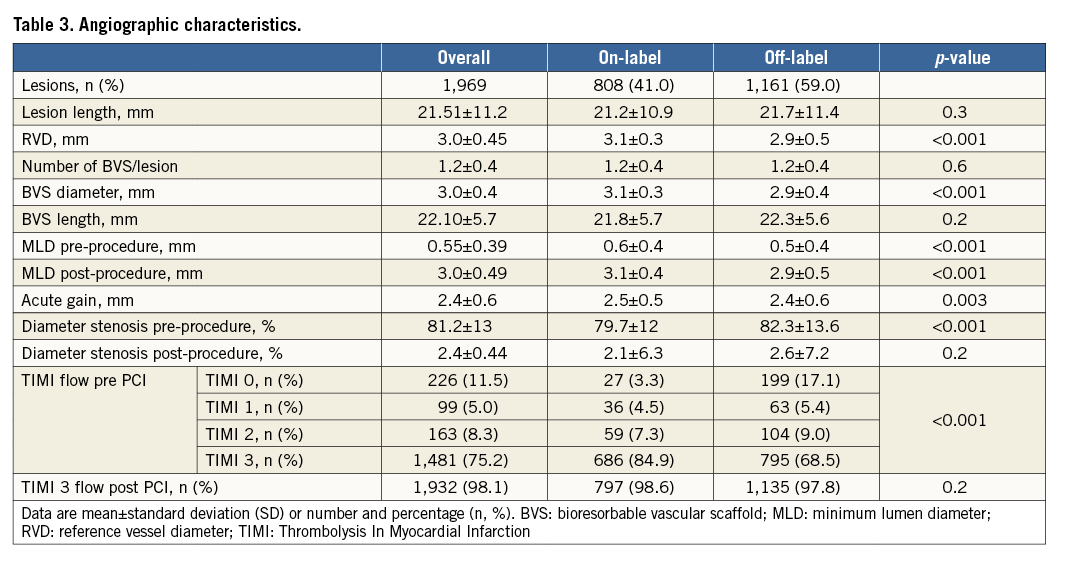
Patients in the off-label group had a higher SYNTAX score (11.6±6.8 vs. 12.6±7.3, p=0.001) and received a stronger antiplatelet regimen compared to their counterparts.
CLINICAL OUTCOMES
The 30-day outcomes have been extensively reported elsewhere12.
One-year follow-up was available for 96% of the eligible population. The TLR rate was 3.3% and the ScT rate was 1.3%. Cardiac death occurred in 0.5% of patients. The DOCE rate was 3.9% (Table 4). All the ScT occurred while the patients were on DAPT. Details of patients experiencing definite or probable ScT are reported in Supplementary Table 1.
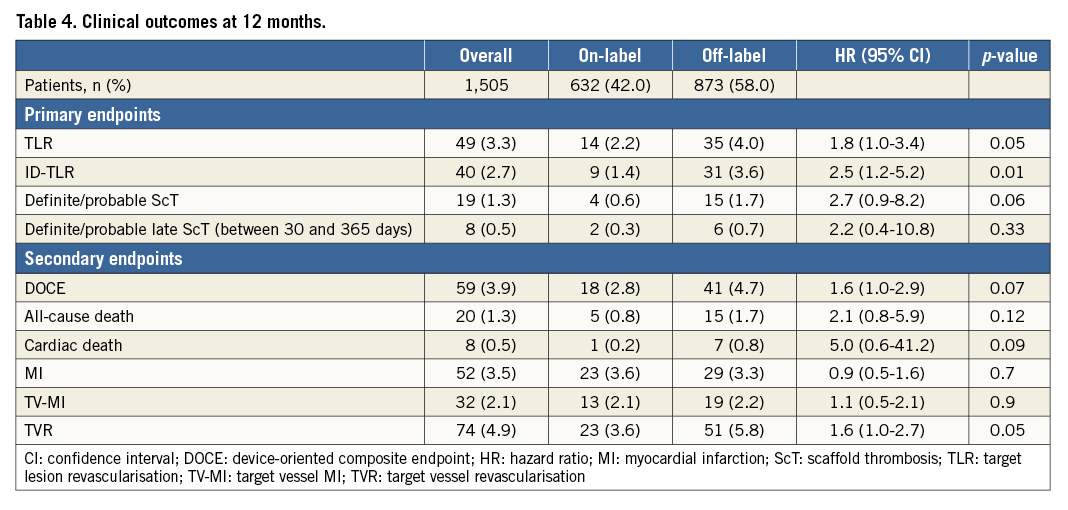
Of note, the TLR rate was significantly higher in the off-label (4.0%) compared to the on-label (2.2%) group (HR 1.8, 95% CI: 1.0-3.4; p=0.05), while a persistent trend towards a higher rate of ScT was reported in the off-label group (1.7% vs. 0.6%; HR 2.7, 95% CI: 0.9-8.2; p=0.06). Also, there was a trend towards a higher DOCE rate in the off-label compared to the on-label group (4.7% vs. 2.8%; HR 1.6, 95% CI: 1.9-2.9; p=0.07) (Table 4). Kaplan-Meier curves showing the incidence of 12-month TLR and ScT in the two groups are shown in Figure 1 and Figure 2. Of interest, no differences were reported in terms of primary outcomes between patients treated only with BVS vs. BVS plus other stents (TLR: 2.8% vs. 4.2%, p=0.2; definite/probable ScT: 0.9% vs. 1.4%; p=0.3), while higher cardiac death (0.3% vs. 1.1%, p=0.05) and MI (2.8% vs. 4.9%; p=0.05) rates were observed in the “hybrid” group.
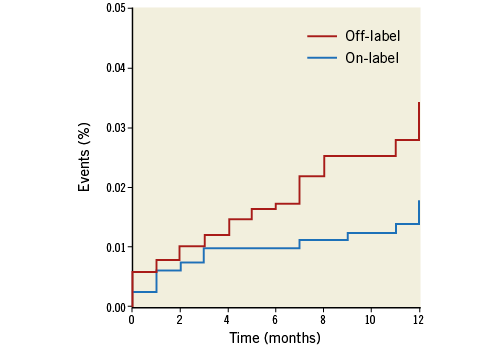
Figure 1. Kaplan-Meier curves showing the incidence of target lesion revascularisation in the “on-label” vs. “off-label” groups at one-year follow-up.
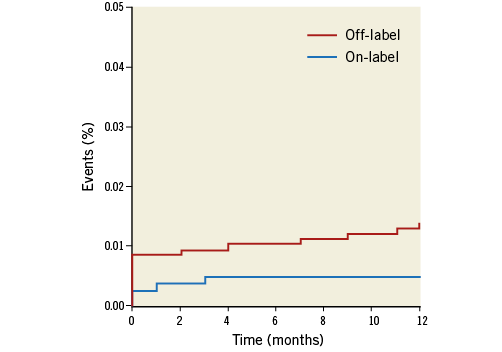
Figure 2. Kaplan-Meier curves showing the incidence of scaffold thrombosis in the “on-label” vs. “off-label” groups at one-year follow-up.
PREDICTORS OF TLR IN THE OFF-LABEL POPULATION
In order to identify which of the off-label lesion characteristics was a predictor of TLR, a subgroup analysis was performed in this population. Using Cox regression analysis, all variables were tested in an univariable model with regard to the prediction of TLR. In the multivariate Cox regression, age lost its significance (HR 1.03, 95% CI: 0.9-1.1; p=0.06), while ISR (HR 3.28, 95% CI: 1.5-7.1; p=0.003), CTO (HR 4.05, 95% CI: 1.7-9.7; p=0.002) and BVS diameter (HR 0.26, 95% CI: 0.1-0.7; p=0.006) remained independent predictors of TLR (Supplementary Table 2).
Discussion
The main findings of this registry aiming to assess the clinical outcome following a successful, unrestricted BVS implantation in real-world patients with coronary artery disease (CAD) are:
1. Low rates of TLR and ScT (3.3% and 1.3%, respectively) during one-year follow-up;
2. Low rate of DOCE (3.9%) during one-year follow-up;
3. Higher rates of predilatation and BVS post-dilatation (compared to other registries) may have influenced the one-year outcomes.
BRS were introduced as a technological advance to eliminate permanent caging of the vessel wall by metallic prosthesis with hypothetical advantages to be determined at very long-term follow-up2. However, data from randomised studies have raised doubts on the early and long-term BVS performance when compared to the X-EES. In particular, a similar one-year clinical efficacy as well as increased rates of TV-MI and ScT have been reported3,4. More recently, the ABSORB II trial highlighted an excess in TV-MI and ScT at three-year follow-up compared to X-EES5. To what degree the implantation technique, the BVS per se or the subset of lesions/patients treated contributed to the scaffold failure is still under debate.
In this context, the RAI registry has the specific feature of relatively low exclusion criteria. In 58% of our patients, a BVS was implanted in at least one off-label subset. The possibility of assessing the BVS performance in such scenarios was under-represented in prior studies7.
Notably, ACS patients were excluded from the largest ABSORB trial, ABSORB III13, while they represented 47% of the whole population of the GHOST-EU registry (GHOST-EU)14, 53.6% in the all-comers AIDA trial15, and 59% in ours. Furthermore, despite the fact that indications for an aggressive lesion preparation and BVS post-dilatation were only suggested (not mandatory or pre-specified in terms of balloon type or atmospheres), the operators’ behaviour was surprisingly in line with the actual standards for an optimal BVS implantation, with very high predilatation and post-dilatation rates. These technical steps during BVS implantation resulted in being different in our study compared to the GHOST-EU registry (predilatation: 100%, post-dilatation: 49%)14, ABSORB III trial (predilatation: 100%, post-dilatation: 65.5%)13 and AIDA trial (predilatation: 100%, post-dilatation: 63%)15. Along with high predilatation and post-dilatation rates, 48% of our patients (all with ACS at admission) received a novel antiplatelet agent (i.e., ticagrelor and prasugrel) as a part of the DAPT regimen. This adjunctive pharmacological aspect (using newer drugs) may have played a protective role against sudden early and late thrombotic events, just as DAPT prolongation could have done beyond one year for very late events16.
As a potential consequence of those procedural approaches, TLR, ScT as well as DOCE rates (3.3%, 1.3%, and 3.9%, respectively) were lower in comparison to those of similar studies on this topic. In particular, the multicentre GHOST-EU retrospective registry included a large variety of patient/lesion subsets (1,189 patients/1,440 lesions) showing TLR and definite/probable ScT rates of 2.5% and 2.1% at six-month follow-up. The authors commented that at least a certain number of events could be attributable to procedural issues including dissections left untreated and inadequate BVS apposition or expansion. They also commented that 39% of the patients who experienced ScT had the device post-dilated while fewer than 15% had intravascular imaging14.
Similar results were shown in the ISAR-ABSORB study17, where the rates of definite/probable ScT and TLR were 2.6% and 9.1% at 12 months. As the bulk of ScT were seen within 30 days, this suggested that the occurrence of events is probably related to specific procedural issues (post-dilatation: 71.5%).
The importance of the implantation technique to obtain a symmetrical and optimal BVS expansion reducing the shear stress and the risk of early and midterm events has definitely been demonstrated by different studies7,18. Puricel et al demonstrated an ScT rate of 1.0% at one year7, while in the single-centre, real-world analysis by Tanaka et al the ScT, TV-MI and TLR rates at one year were 1.2%, 1.8% and 6.6%, respectively18. Our clinical findings are in line with those results, even if the intravascular imaging-guided BVS implantation rate was lower in our cohort compared to the study by Tanaka et al (12.4% vs. 85.8%); this aspect could have influenced the outcomes particularly in off-label lesions. On the other hand, the significantly higher cardiac death and MI rates reported in the “hybrid” group (no data for this population reported by Tanaka et al) may reflect our more cautious lesion selection (according to the limitations of current BVS) resulting in DES plus BVS use for the management of very complex CAD containing unfavourable BVS segments.
We were also able to identify ISR, CTO and BVS diameter as significant predictors of TLR at one year. Even if previous studies demonstrated the immediate feasibility and later acceptable clinical performance of BVS in both ISR19 and CTO20, on the basis of our results it seems inadvisable to consider BVS as the first-line treatment for these complex subsets, particularly if a small diameter BVS is required. Indeed, even BVS diameter was significantly associated with TLR at follow-up in our study. This finding indirectly confirms the actual concerns regarding BVS implantation in small vessels because of the risk of a higher BVS footprint (compared to larger vessels), potentially leading to low post-procedural MLD and BVS failure7. The evidence from our study further highlights that a proper patient/lesion selection (i.e., carefully evaluating BVS implantation in off-label scenarios such as vessels with RVD <2.5 mm, ostial lesions, heavily calcified lesions, CTOs, complex bifurcations, ISR, ULM and some kinds of ACS and long lesions), along with scaffold implantation techniques, may affect clinical outcomes in BVS-treated patients with a higher impact than that known for the highly performing DES.
However, whether an optimal BVS implantation strategy may impact on the long-term (>1 year) outcome is still unknown.
Limitations
The observational nature of the study and the lack of some procedural data (i.e., predilatation balloon type or size, the relation between the pre-PCI RVD and balloon size, dilatation pressures) represent the main limitations of the study. The choice of using a BVS instead of another device was left to the operators’ discretion, depending on their own experience and on device availability. QCA evaluation was performed by every single operator (no core lab analysis) during (on-line) or after (off-line) implantation, thus potentially limiting the uniformity of the assessment and subsequently the quality of the data reported. Also, the SYNTAX score was calculated on-site and off-line by different operators. Furthermore, the comparison of outcomes between on-label vs. off-label is unadjusted and only hypothesis-generating.
Conclusions
Our data from a real-world population suitable for scaffold implantation suggest that BVS use is associated with acceptable one-year clinical outcome when systematic lesion preparation and device post-dilatation are applied. However, a higher rate of adverse events was observed when this device was used in off-label lesions. The long-term follow-up of the RAI registry (>1 year) will perhaps confirm these favourable midterm outcomes.
| Impact on daily practice The one-year clinical outcome of the RAI registry supports the careful use of BVS for the treatment of on-label indications (according to the manufacturer’s IFU) using a low threshold for lesion predilatation and device post-dilatation. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Details of patients with definite or probable scaffold thrombosis.
Supplementary Table 2. Predictors of TLR in the off-label population.
To read the full content of this article, please download the PDF.

