
During the period of evolution from balloon percutaneous transluminal coronary angioplasty (PTCA) to bare metal stents (BMS) and drug-eluting stents (DES), early and late adverse clinical event rates have progressively declined1. Although device iterations including novel alloy composition, reduced strut thickness and improved polymer biocompatibility and/or resorption further improved clinical outcomes to one year, beyond this time point even the “best” metallic DES are associated with a 2-4% annualised rate of target lesion failure (TLF; a composite of cardiac death [CD], target vessel myocardial infarction [TVMI] or ischaemia-driven target lesion revascularisation [ID-TLR]) in 5- to 10-year follow-up, similar to rates observed following BMS or first-generation DES2,3,4. A unifying mechanism for similar annualised event rates regardless of device is the common presence of a metallic implant which mechanically distorts and constrains the vessel, preventing vasomotion and adaptive coronary remodelling. A persistent metal scaffold serves as a nidus for inflammation, neoatherosclerosis or stent fracture – with consequent thrombosis and/or restenosis. Early observational studies suggested very late (≥1 year) clinical benefit for a “leave nothing behind” percutaneous coronary intervention (PCI) strategy. In patients treated with PTCA, BMS or first-generation DES for ST-segment elevation MI, stents reduced the rates of stent/lesion thrombosis and target vessel reinfarction up to one year5. However, 1- to 10-year landmark analyses demonstrated a reduction in these adverse events favouring PTCA. This was attributed to the absence of a persistent metal scaffold. Bioresorbable scaffolds (BRS) were developed to provide mechanical scaffolding, drug delivery and clinical outcomes similar to contemporary metallic DES up to one year, with improved longer-term outcomes due to restoration of adaptive remodelling and the absence of metal fracture-related adverse events following resorption6. Randomised controlled trials (RCT) supporting regulatory approval of the bioresorbable vascular scaffold (BVS; Abbott Vascular, Santa Clara, CA, USA) demonstrated non-inferiority to everolimus-eluting DES (EES) using a composite endpoint (TLF) reflecting device effectiveness and safety. These trials (ABSORB II, III, Japan, China) enrolled “low-risk” patients with non-complex target lesions, without protocol-driven optimised implantation techniques (OIT)6,7. In this issue of EuroIntervention, Smits et al report one-year outcomes from the ABSORB-COMPARE RCT comparing BVS to EES in more complex patient and lesion subsets8.
Despite protocol-specified OIT for BVS (and trial primary endpoint achievement), ABSORB-COMPARE was terminated prematurely due to higher rates of TVMI and scaffold thrombosis (ST) following BVS implantation. Similarities in clinical and angiographic measures are evident across BVS RCT which reflect limitations intrinsic to device (BVS) and trial design. First, BVS strut thickness creates abnormalities in peri-strut shear stress distribution and thrombogenicity with increased ST rates across patient and lesion risk profiles9. ST hazard is magnified in small (≤2.5 mm) vessels due to exponential increases in abluminal strut surface area (device footprint) and strut volume/vessel volume ratio10. Second, strut thickness and device profile significantly reduced BVS delivery and device success. Third, despite more frequent predilatation and post-dilatation in the BVS group, post-procedural angiographic measures consistently favoured EES (>minimum lumen diameter and acute gain; lower % residual stenosis). Fourth, although the BVS was consistently non-inferior for TLF up to one year by pre-specified margins, point estimates consistently favoured EES, and rates of TVMI and ST were increased with BVS. Pooled individual patient data up to three years (time point for complete BVS resorption) demonstrated increased rates of TLF, TVMI and ST following BVS implantation7. Intravascular imaging (1-3 years) revealed BVS-specific mechanism(s) for device thrombosis/restenosis related to polymer bulk erosion, late strut discontinuity and prolapse of scaffold fragments into the vessel lumen (intraluminal scaffold dismantling)11. Fifth, beyond complete BVS resorption (three years) comes the “calm after the storm” with differences in treatment effect by device over time for TLF (pint=0.046) and ST (pint=0.03) which support the “leave nothing behind” concept7.
What’s new in the ABSORB-COMPARE one-year report? First, TLF rates are low for both devices, despite increased clinical and angiographic complexity. Increased complexity is reflected in higher rates of ST for both devices, despite protocol-driven OIT. ST hazard after BVS persists in ABSORB-COMPARE, despite better target lesion evaluation and preparation (increased intravascular imaging and lesion predilatation), shorter lesions, fewer small vessel lesions, less frequent device overlap and more frequent high-pressure post-dilatation with non-compliant balloons, and was sustained up to one year without mitigation by OIT. This observation suggests that iteration(s) in BVS (thinner struts, different mechanism of resorption) are required to reduce ST hazard. Second, the pre-specified non-inferiority margin of 4.5% for one-year TLF exceeds the observed TLF rate with the control (EES) device, confounding conclusions of non-inferiority. Third, despite “non-inferiority” for TLF at one year, increased rates of ST and TVMI question the reliability of this endpoint to support device regulatory approval. Finally, as clinical follow-up beyond five years was suspended in ABSORB III, planned seven-year follow-up in ABSORB-COMPARE can provide insights regarding incremental clinical benefit for BVS following resorption. Further divergence in adverse events favouring BVS could renew interest in BRS and prompt iterations focused on mitigation of early hazards.
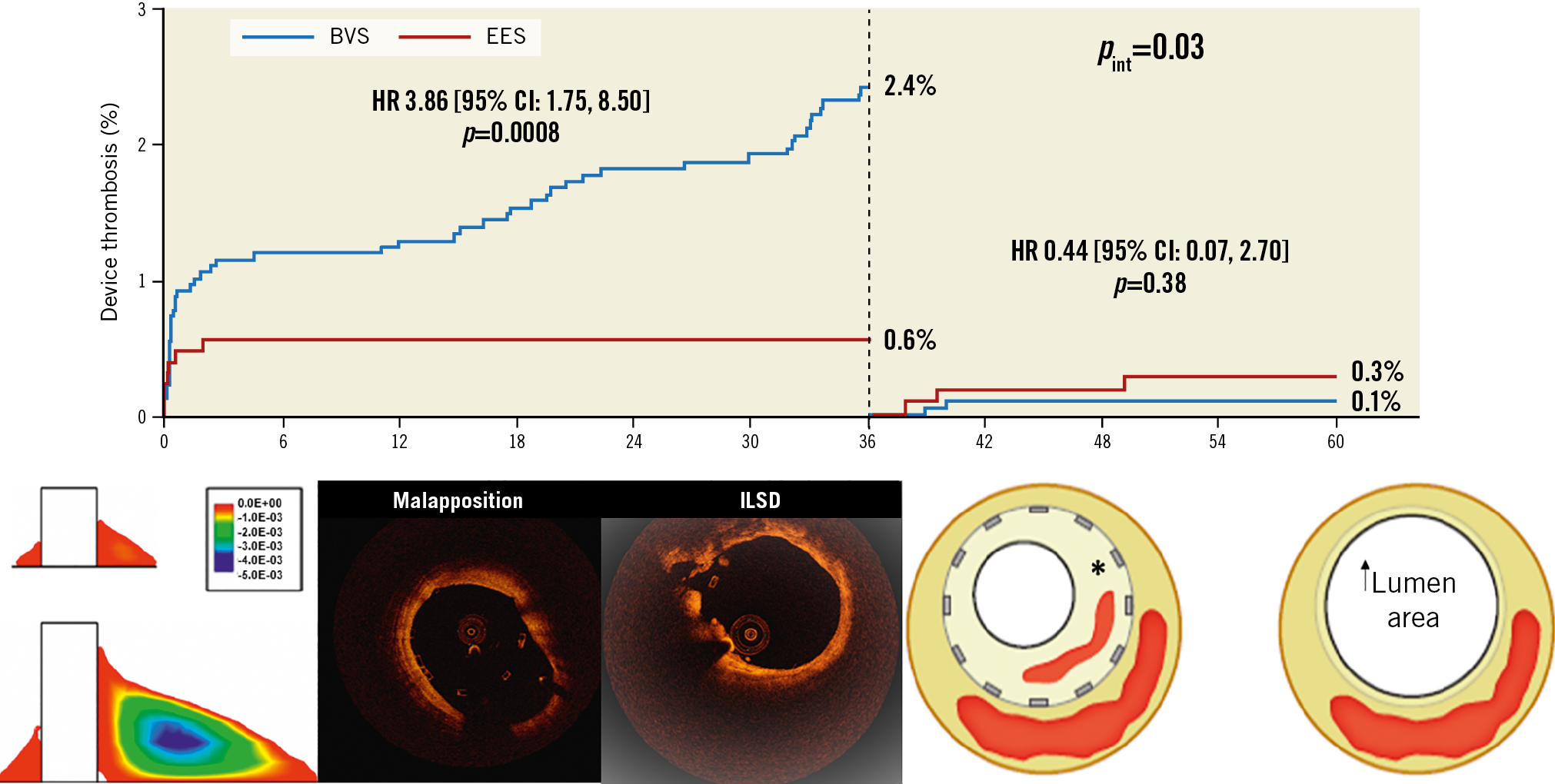
Figure 1. Biphasic nature of BVS relative hazard for device thrombosis over time. Hazard is increased (<36 months) largely due to strut thickness, polymer physical characteristics and resorption properties (intraluminal scaffold dismantling [ILSD]). Hazard is reduced
(>36 months) following device resorption and positive adaptive vessel remodelling. *neointimal hyperplasia, neoatherosclerosis and thrombosis with lumen loss after metallic DES. Modified with permission from Kereiakes et al6, Stone et al7 and Kolandaivelu et al9. OCT images courtesy of Ziad Ali, MD.
Conflict of interest statement
D. Kereiakes reports receiving consulting fees from Sino Medical Sciences Technology, Inc., Boston Scientific Corporation, Elixir Medical, Inc., Svelte Medical Systems, Inc. and Caliber Therapeutics/Orchestra Biomed.
Supplementary data
To read the full content of this article, please download the PDF.

