Introduction to the session
The aim of the article is to capture the session at EuroPCR 2016, communicate the analysis of the trialists, and report the views expressed in the interactive discussion. The article does not constitute an independent review of the topic by the authors. The session focused on whether the ABSORB III trial will change clinical practice1.
Background: what was known before ABSORB III?
A clear overview was presented of the available data before the ABSORB III trial1 in which we were reminded that, at the time the ABSORB III study was in draft, virtually the only evidence were the results from ABSORB cohort A3 (with up to five years of clinical follow-up) and ABSORB cohort B4 (two-year follow-up). Additionally, there was some early evidence (“a two-year interim snapshot”) in 450 patients included in ABSORB EXTEND5. In aggregate, these single-arm exploratory studies demonstrated the feasibility of BRS in simple patients and lesions. The five-year clinical outcome from cohort A showed a very low MACE (n=1 patient, 3.4%) rate, which was confirmed by both ABSORB cohort B (n=3 patients, 6.8%) and the interim analysis from ABSORB EXTEND (n=33 patients, 7.3%). It is of note that a single patient suffered from ARC-defined definite/probable scaffold thrombosis in this first analysis of 523 patients with a mean follow-up of 2.2 years. Moreover, indirect comparison of MACE in patients included in ABSORB cohort B and ABSORB EXTEND with SPIRIT I, II & III suggested a small advantage of EES over BRS in the very early follow-up (13 months in ABSORB cohort B and four months in ABSORB EXTEND), followed by a reduced rate of MACE in the BRS compared to EES. An indirect comparison was presented on angina status one year after implantation of BRS (n=337 patients from ABSORB EXTEND) compared with EES (SPIRIT IV, n=2,000). This analysis suggested a lower incidence of angina after BRS than EES. Therefore, at the time ABSORB III was designed, the available evidence suggested that BRS was feasible (although good lesion preparation, accurate sizing and post-dilatation were mandatory), safe and may be associated with less chest pain.
At the time ABSORB III was finalised >20,000 patients with BRS were included in trials and the number of publications boosted. In aggregate, two randomised controlled trials comparing BRS with DES demonstrated reassuring performance of BRS. When EverBio II6 with minimal exclusion criteria demonstrated satisfactory angiographic results of BRS compared to EES/BES, the in-segment late lumen loss –a surrogate marker of efficacy– was however slightly but significantly higher in BRS compared to EES/BES. The ABSORB II7 demonstrated similar clinical outcomes at one year in BRS compared with EES and lower cumulative rates of “first new or worsening” angina in BRS than in EES. Finally, the publication that had the most impact during this period was the European GHOST-EU registry8 that revealed a high cumulative incidence of scaffold thrombosis (2.1%) at six months.
The case presentation
A case was presented in order to illustrate a patient who could have been included in the ABSORB III trial. The patient was a 59-year-old male who was planned for PCI of a tight but short stenosis in the mid right coronary artery (RCA) (Figure 1). Optical coherence tomography (OCT) imaging demonstrated mainly a fibrotic pattern without significant calcification. The patient was under dual antiplatelet therapy with aspirin and prasugrel.
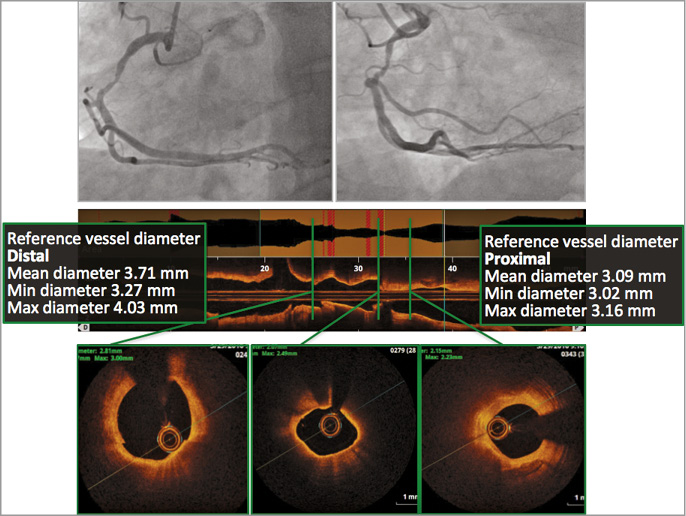
Figure 1. Right coronary angiogram and OCT imaging of a 69-year-old male with staged PCI of four weeks after PCI of the LAD. The OCT imaging demonstrated a focal lesion with mainly a “fibrotic” OCT pattern and without significant calcification. Proximal reference vessel diameter was 3.7 mm.
The audience was polled about the choice of BRS in a relatively young patient with a focal and non-calcified lesion. Most of the attendees would not have treated this patient with BRS, which was mainly due to the limited availability of BRS at their institutions
Trial analysis: summary of the trialist’s critical review
The ABSORB III study, published in the New England Journal of Medicine, was a large-scale, multicentre, mostly US, single-blind, head-to-head comparison of Absorb BRS against the conventional XIENCE EES with a 2:1 randomisation. The primary endpoint was target lesion failure (TLF) at one-year follow-up. The study was designed and powered to demonstrate non-inferiority of BRS over EES. The study was also powered for the secondary endpoints angina at one year, ischaemia-driven target vessel revascularisation and all revascularisation. The study was supported by Abbott Vascular. The concept of a non-inferiority study with the null hypothesis (TLFEES - TLFABSORB ≥4.5%) with six scenarios of results (Figure 2) was explained and how this impacts on the delta margin value. Also an explanation was given for the reason for choosing a delta margin of 4.5% based on results from the random effect meta-analysis that was explained in the appendix of the NEJM article and was based on an average delta in TLF rates between the bare metal stent group and first-generation DES (11.6% [90% CI: 7.8%, 15.4%]) and between first-generation DES and second-generation DES (2.5% [90% CI: 1.2%, 3.7%]) using the DerSimonian and Laird method, according to a methodology recommended by the US Food and Drug Administration (FDA). Based on the TLF rate found in the EES group of the ABSORB III trial at one year (6.1%), the audience was asked whether a hypothetical 10.6% TLF rate is considered as clinically acceptable. The results were then summarised. For the primary endpoint TLF: in the “intention-to-treat” analysis, the difference between BRS (TLF 8.0%) and EES (6.1%) was 1.7% [–0.5%, 3.9%], pnon-inferiority=0.007, which numerically favours EES but still meets non-inferiority. Since many crossovers happened during the study – mostly BRS to EES (n=55), the “as-treated” analysis was still non-inferior (pnon-inferiority=0.01) but closer to the 4.5% margin with BRS (TLF 7.8%) and EES (6.1%) and a delta value of 1.9% [–0.3%, 4.1%]. These results are not generalisable since the population was highly selected, typical of young patients with a stable condition and non-complex coronary lesions. Indeed, from 13,789 patients screened, only 14.8% were randomised because most patients (n=10,690) did not meet the angiographic eligibility criteria. With 193 sites and an inclusion period of 12 months, about one patient was included per month and per centre. Post-dilatation was performed in 60% and intravascular guidance in 11% of cases. The crude results showed less device success and longer procedures with BRS than EES, larger devices used and more post-dilatation, but poorer immediate angiographic results with BRS. There was a ≈2% increase in TLF with BRS in the “as-treated” analysis, and all point estimates for each of the primary outcome components numerically, albeit not significantly, favoured EES. The risk of thrombosis was twice as high in BRS with a significantly higher rate of subacute thrombosis despite a higher adherence to new P2Y12 inhibitors in the BRS group. Small lesions (RVD <2.25 mm) were found in 18% of cases whereas this was an exclusion criterion, and the incidence of scaffold thrombosis was at its extreme (up to 8.1% in lesions without post-dilatation) in this particular subset of lesions.
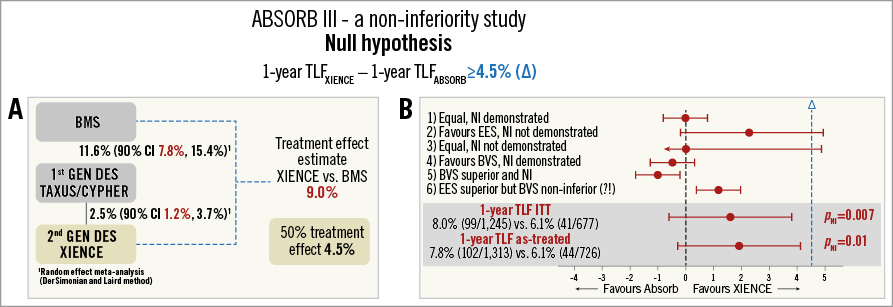
Figure 2. Concept behind a “non-inferiority study”. A) Explanations about the way the authors chose an average delta margin of 4.5% in TLF rates based on results between the bare metal stent group and first-generation DES (11.6% [90% CI: 7.8%, 15.4%]) and between first-generation DES and second-generation DES (2.5% [90% CI: 1.2%, 3.7%]) using the DerSimonian and Laird method mandated by the US Food and Drug Administration (FDA). B) Six different scenarios found in the “non-inferiority study” and results from the primary outcome in perspective (bottom).
Discussion and audience interaction
This latter evidence produced a lively discussion between the panellists and the audience to ascertain whether these particular cases should have been excluded due to protocol violation. This was considered to be part of the learning curve and was considered crucial information. The further discussion then touched on the general acceptance of an initial inferiority of the BRS over the EES and whether BRS would demonstrate a clear superiority in the long run. Consequently, even if it was not the purpose of the study to demonstrate non-inferiority to this time point (even if the curves were strictly superimposed), there was hope for BRS superiority in longer follow-up. Therefore, the audience considered that one should put a positive spin on it.
The case resolution and the practitioner’s view
The treatment of the patient was summarised (Figure 3). He underwent OCT analysis of the lesion before lesion preparation with an NC balloon. Due to a partial underexpansion of the balloon (in two orthogonal projections), he then moved to advanced lesion preparation with a scoring balloon which permitted full balloon expansion and BRS implantation. Post-dilatation was performed with OCT guidance and with good final angiographic and OCT results. The importance of a BRS-dedicated implantation technique and the use of OCT guidance in selected cases was highlighted.
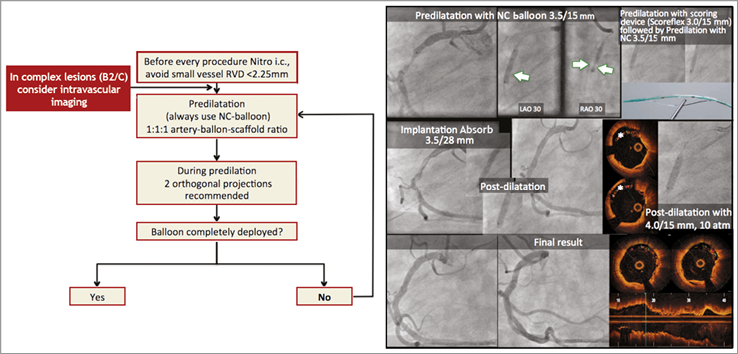
Figure 3. Percutaneous coronary intervention in the right coronary artery using a dedicated implantation protocol (right-hand side), OCT guidance and post-dilatation. *demonstrates incomplete scaffold apposition.
Conclusion by the Chairperson: where do we stand now?
The session was concluded by how this study will impact on our practice: proper patient and lesion selection is of the utmost significance in BRS, and BRS are probably not indicated in small vessels. The importance of good lesion preparation, post-dilatation and a liberal use of intravascular imaging was also re-emphasised. The educational analysis of the study finally illustrated how the concept of non-inferiority might be complex and could lead to counterintuitive results.
Conflict of interest statement
The authors have no conflicts of interest to declare.

