
Introduction
The Absorb™ bioresorbable everolimus-eluting scaffold (Abbott Vascular, Santa Clara, CA, USA) was designed to provide a transient vessel scaffold and everolimus elution followed by bioresorption of the polymer, aiming to restore coronary structure and functionality. Although randomised controlled trials comparing the bioresorbable scaffold with the XIENCE everolimus-eluting stent (Abbott Vascular) demonstrated non-inferiority of Absorb compared with XIENCE in target lesion failure (TLF) at one year, the excess of early, late, and very late scaffold thromboses raised safety concerns about the bioresorbable scaffold, leading to a halt of commercialisation of the product. Theoretically, device-related events should diminish after the completion of the bioresorption process (three years); however, clinical data beyond four years have not yet been reported in the context of the randomised controlled ABSORB II trial1. The objective of the current report is therefore to present comparative clinical results of the Absorb scaffold and XIENCE stents up to five years in this randomised trial.
Methods
Details of the study design, study device, procedure and clinical follow-up have been published elsewhere2. Briefly, 501 patients were randomly assigned to receive either the Absorb scaffold or the XIENCE stent in a 2-to-1 fashion. The primary endpoints were assessed by angiography at three-year follow-up. At the three-year visit, patients were re-consented for extended follow-up up to five years. Twenty patients refused to participate in the extended follow-up. Also, at five years, thirty-seven patients were removed early due to a lapse in the protocol renewal by the Polish Ethics Committee. All clinical events were adjudicated by an independent clinical event adjudication committee. Fisher’s exact test was used to compare categorical variables. The Kaplan-Meier method was used to estimate the cumulative rates of events and the log-rank test was performed to examine the differences between groups.
Results
Five-year follow-up was available in 256 patients (76.4%) and 125 patients (75.3%) in the Absorb arm and the XIENCE arm, respectively. Between four and five years, 2 patients died and 3 patients were lost to follow-up. Twelve patients came back for five-year follow-up, but before the predefined five-year time window. Details of the patient flow are shown in Figure 1. Between four and five years, TLF was observed in 0.8% (2 patients) and 0.0% (0 patients) in the Absorb arm and the XIENCE arm, respectively (p=0.33). Also, myocardial infarction was observed in one patient in the XIENCE arm (0.8%, p=0.33) between years 4 and 5 of follow-up. The patient-oriented composite endpoint (PoCE) was observed in 3.7% (10 patients) and 2.3% (3 patients) in the Absorb and the XIENCE arm, respectively (p=0.46). Importantly, no definite stent or scaffold thrombosis was observed between four and five years in either arm.
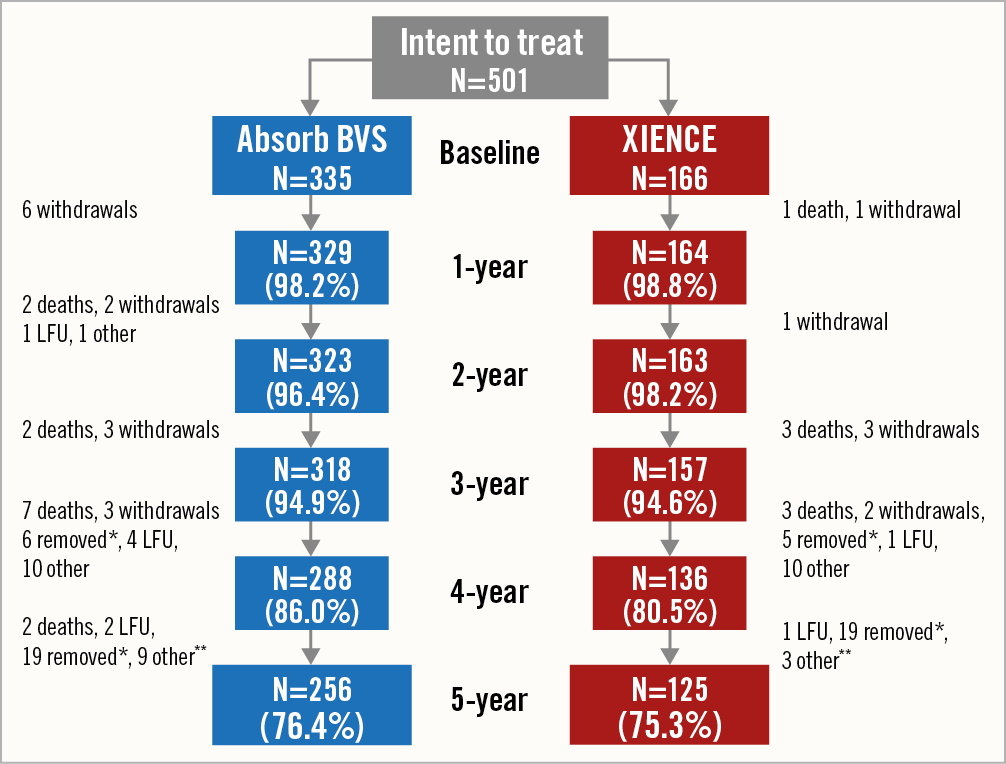
Figure 1. Flow chart of the ABSORB II study at five-year follow-up. LFU: lost to follow-up. * The Polish Ethics Committee approval of the ABSORB II trial ended on 31 December 2016. Considering this, subject data past 31 December 2016 from the Poland site were removed from the 5-year ABSORB II analysis. ** Between 4 and 5 years, 12 patients were counted as “other.” 1 patient refused to return for the 5-year visit. There were 11 patients who had completed 4-year visits but their 5-year visits occurred before the allowable window and therefore they are counted as removed without a 5-year visit.
The cumulative TLF rate was 11.8% (38 patients) and 5.6% (9 patients) in the Absorb arm and the XIENCE arm, respectively (p=0.033), whereas the five-year PoCE rate was 25.0% (80 patients) and 25.7% (41 patients), respectively (p=0.78) (Figure 2). At the five-year visit, 17.8% and 12.7% of patients were on dual antiplatelet therapy (DAPT) (p=0.14), although the majority of patients remained on aspirin (72.2% and 69.3%, p=0.50). Individual components of the composite endpoint and a non-hierarchical analysis of clinical outcomes are presented in Supplementary Table 1.
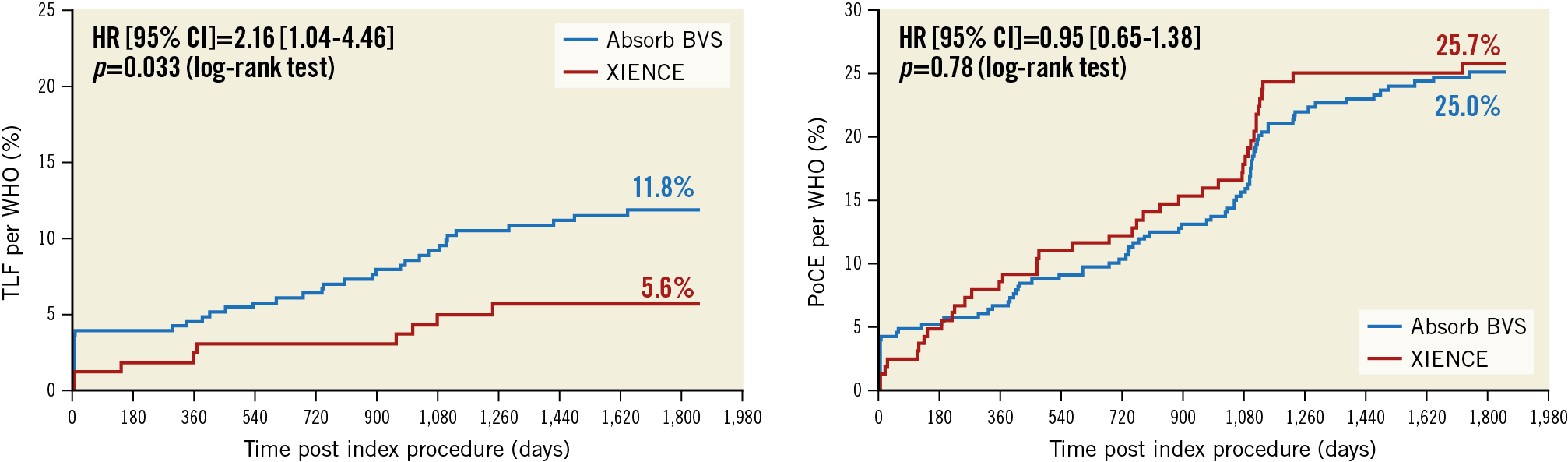
Figure 2. Target lesion failure and patient-oriented composite endpoint up to five years. TLF (target lesion failure): cardiac death, target vessel myocardial infarction, and clinically indicated target lesion revascularisation. PoCE (patient-oriented composite endpoint): death, any myocardial infarction, and all revascularisation. Myocardial infarction was adjudicated according to the WHO definition. HR: hazard ratio; CI: confidence interval; BVS: bioresorbable vascular scaffold; WHO: World Health Organization
The risk difference between the Absorb and XIENCE arms in terms of TLF and PoCE at five years was evaluated according to several baseline and lesion characteristics, as shown in Supplementary Table 2 and Supplementary Table 3.
Discussion
The main findings of this extended follow-up of the ABSORB II trial are: i) there was no additional device thrombosis between four and five years; ii) the device-oriented composite endpoint and the PoCE remained low in both arms.
In previous randomised trials comparing Absorb and XIENCE, Absorb reached the primary endpoint in terms of TLF; however, the increased rate of scaffold thrombosis up to three years was considered to be a safety concern. The device was therefore withdrawn from commercial sale. Some studies planned to continue clinical follow-up up to seven years (e.g., COMPARE ABSORB) to investigate the potential very long-term benefit of the Absorb scaffold versus XIENCE metallic stents. The current results are encouraging in that very long-term risks of scaffold thrombosis and TLF are diminishing after completion of the degradation of the bioresorbable scaffold (3 years). Indeed, the five-year results of the ABSORB III trial also indicated the safety of the Absorb BVS beyond three years after implantation3.
The Absorb bioresorbable scaffold was designed to be bioresorbed by three years. Gas chromatography analysis in preclinical models demonstrated that, by 36 months, poly-l-lactide (PLLA) becomes undetectable4. The healing process of the vessel continues after biodegradation of polymer with connective tissues infiltrating into the histological void, previously occupied by the polymer5. At four years, the integration of the scaffold is almost complete, which is reflected in the surge of light intensity imaged by optical coherence tomography (OCT)6. Although case reports have described persistence of struts beyond three years, preclinical studies have indicated that such findings probably reflect only a delayed cellularisation of the matrix occupying the resorbed scaffold struts6. It was previously hypothesised that beyond the point of resorption the vessel would recover its physiological capacity and its native structure. Five-year results of the first-in-man ABSORB cohort B study, in which bioresorbable scaffolds had been implanted in simple stenotic lesions, showed low restenosis and low major adverse cardiac event rates, especially after the first three years7. Invasive imaging follow-up studies up to five years demonstrated stable lumen dimensions from midterm (24 or 36 months), suggesting the long-term efficacy of the bioresorbable scaffold in a selected population7. In the current ABSORB II trial, the absence of scaffold thrombosis and diminished event rates between three and five years are in line with the observations made in animal studies.
Limitations
This study has several limitations. The study was not powered for the clinical endpoint, and hence these data remain hypothesis-generating. Since the original study design was limited to follow-up up to three years, 20 patients refused to participate in this extended follow-up at the time of re-consenting. Imaging follow-up at three years might have contributed to the increase of PoCE in both arms at around that time.
Conclusion
In conclusion, this extended follow-up of the randomised ABSORB II trial demonstrates the absence of scaffold/stent thrombosis from four years to five years, and very low additional events beyond three years, the time point of full scaffold resorption. In the present study, the advantage of a bioresorbable scaffold over a metallic stent was not demonstrated, suggesting that an improved version of the bioresorbable scaffold is needed to justify its clinical use.
|
Impact on daily practice The long-term follow-up of the ABSORB II randomised controlled trial demonstrated the absence of very late scaffold thrombosis between four and five years. |
Funding
The ABSORB II trial (NCT01425281) was funded by Abbott Vascular, Santa Clara, CA, USA.
Conflict of interest statement
P. Serruys is a member of the Advisory Board of Abbott Vascular, and reports personal fees from Biosensors, Micel Technologies, Sino Medical Sciences Technology, Philips/Volcano, Xeltis, and HeartFlow, outside the submitted work. B. Chevalier was a consultant for Abbott Vascular and reports personal fees from Abbott Vascular, during the conduct of the study; he reports other from Colibri, and personal fees from Biotronik, Medtronic, and Terumo, outside the submitted work. M. de Sousa Almeida reports that his institution received fees as part of its participation in the ABSORB II trial but not himself. A. Cequier reports grants and personal fees from Abbott Vascular and Biosensors, grants from Boston Scientific, Biomenco, Cordis, OrbusNeich, and the Spanish Society of Cardiology, personal fees from Biotronik and Medtronic, outside the submitted work. M. Sabaté reports the following conflicts of interest outside the submitted work: consultant for Abbott Vascular and iVascular. S. Windecker has received research and educational grants to the institution from Abbott, Amgen, Bayer, BMS, Boston Scientific, Biotronik, CSL Behring, Medtronic, Edwards, Polares and Sinomed. G. Campo reports grants from Boston Scientific, AstraZeneca, Medis, and Sahajanand Medical Technologies, outside the submitted work. D. Dudek reports having received speaker fees, being on the advisory board of and being an investigator for Abbott. R. Rapoza is a full-time employee of Abbott Laboratories, the sponsor of this trial. N. West is employed by Abbott Vascular. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

